This year’s Nobel Prize in Physiology or Medicine honours ground-breaking science and recognises the essential contribution of animal research. Mary E. Brunkow, Fred Ramsdell and Shimon Sakaguchi were awarded the 2025 Nobel Prize for their work on immune tolerance that was only made possible thanks to mice.
The immune system is a powerful safeguard that protects our body from thousands of different invaders that come in all shapes and forms. It can recognise, attack, and destroy bacteria, viruses, fungi, or cancerous cells. But how does the immune system know what to attack? Why doesn’t it target the body’s own tissues? The way this balance is maintained puzzled immunologists for over a century. Mary E. Brunkow, Fred Ramsdell and Shimon Sakaguchi solved a critical part of the riddle based on observations they made in mice.
The immune system’s own security guard
The thymus is a key gland for immune function. It trains white blood cells called T lymphocytes or T cells to focus on specific targets by adding specialised receptors to them. Our bodies create billions of types of T cell receptors, so that invading pathogens can be detected, even if they have never been encountered before. In the process, however, the body inevitably creates T cell receptors that can attach to – and destroy – parts of its own healthy tissues, potentially causing autoimmune diseases. Luckily the body plans for that.
For a long time, researchers thought that the dangerous, “self-directed” T cells were filtered out in the thymus. In the 1980s, experiments in mice led them to believe that T cells matured in the thymus and underwent a selection process called “central tolerance”, a kind of checkpoint passage that eliminated the unwanted cells. But Shimon Sakaguchi showed that the story was much more intricate.
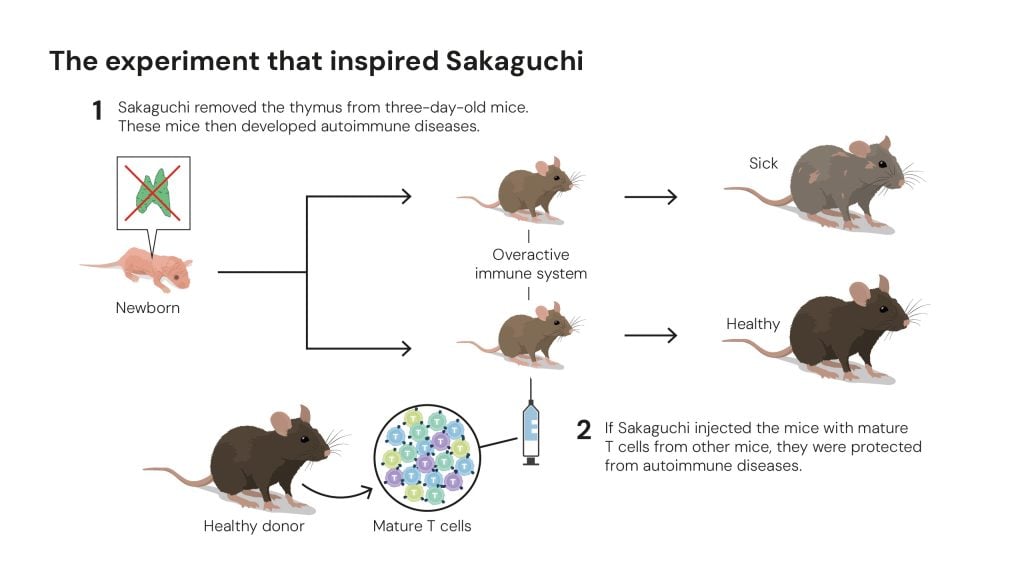
Image above: The experiment that inspired Sakaguchi
© The Nobel Committee for Physiology or Medicine. Ill. Mattias Karlén
An earlier experiment conducted by some of his colleagues had caught his eye. They had surgically removed the thymus of new-born mice, expecting fewer T cells to mature and a weaker immune system to develop. But instead, if the operation took place three days after the mice were born, the immune system did just the opposite and spiked into overdrive, causing a range of autoimmune disorders. Sakaguchi showed that this self-directed immune response could be prevented by injecting the mice with mature T cells from genetically identical mice. This showed that some T cells took on the role of “security guards”, keeping rampaging T cells in check.
A new class of T cells: regulatory T cells
There are several types of T cells with different functions. “Helper” T cells with CD4 proteins on their surface, patrol the body, alerting other immune cells of invading microbes. “Killer” T cells, distinguished by CD8 proteins on their surface, eradicate abnormal or infected cells.
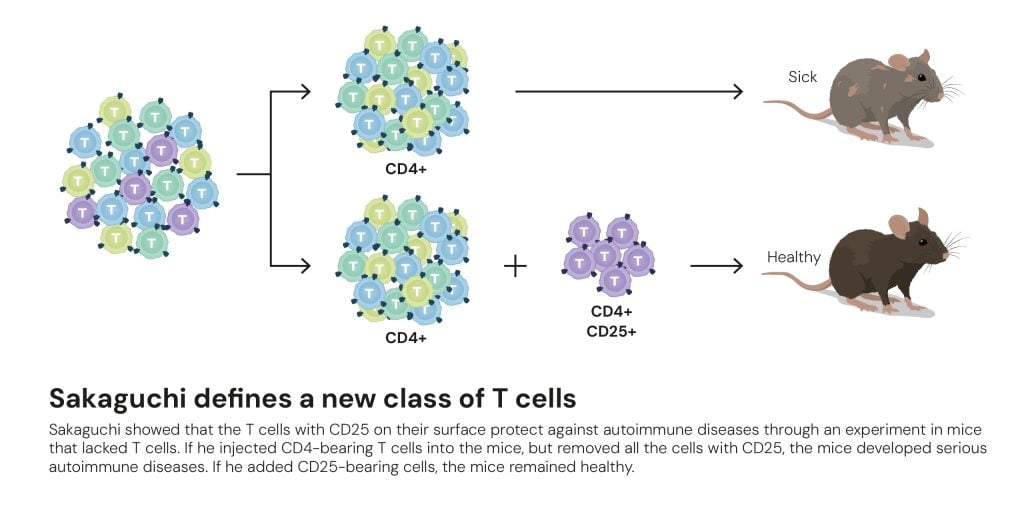
Image above: Sakaguchi defines a new class of T cells
© The Nobel Committee for Physiology or Medicine. Ill. Mattias Karlén
In his experiment, Sakaguchi injected T cells with CD4 on their surface. They should have been helper T cells that wake up the immune system and kick start it, but in the experiment, the immune system was held back instead. His conclusion was that there must be different forms of T cells that also carry CD4. It took Sakaguchi over a decade of research using mice to find this new class of T cell: regulatory T cells, which calm the immune system, and carry CD4 and CD25 on their surface. This was later confirmed by Mary Brunkow and Fred Ramsdell, the two other Nobel laureates.
A mouse model of auto-immune attacks
Mary Brunkow and Fred Ramsdell worked at a biotech company, Celltech Chiroscience, that developed pharmaceuticals for autoimmune diseases. Their work got them interested in a very particular type of mouse: the male scurfy mouse.
The scurfy mouse was unexpectedly born in the 1940s in a US laboratory in Tennessee, that was looking into the consequences of radiation as part of the development of the atomic bomb. The mouse strain captured the researchers’ attention as some male mice were unexpectedly born with scaley and flaky skin, an extremely enlarged spleen and lymph glands, and lived for just a few weeks.
In the 1990s, with the proper molecular tools, researchers we able to investigate why the male scurfy mice got ill. A mutation on their X chromosome was causing a rebellion of their immune system, and their T cells were attacking and destroying their tissues and organs. Brunkow and Ramsdell realised that studying the mouse model could be game changing. If they were able to find the mutant gene and understand the molecular mechanisms underlying the scurfy mouse’s disease, they could gain decisive insights into autoimmune diseases.
The genetics behind an autoimmune disease
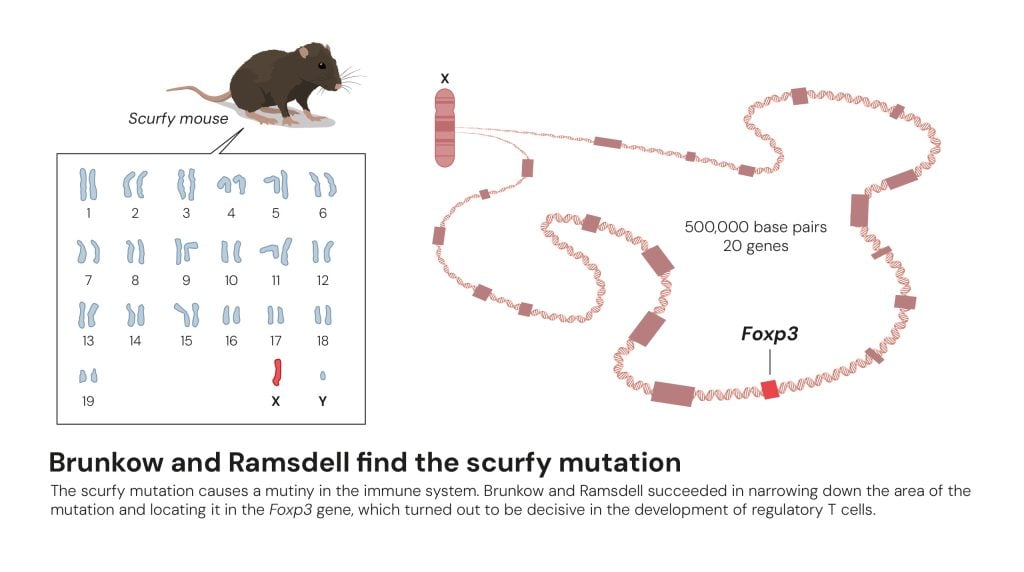
Brunkow and Ramsdell find the scurfy mutation
© The Nobel Committee for Physiology or Medicine. Ill. Mattias Karlén
In the 1990s, looking for a gene took patience and time – a lot of time. Brunkow and Ramsdell went digging, examining gene after gene, looking at and comparing the genomes of healthy mice and scurfy mice, until they found it: the Foxp3 gene.
During their work, Brunkow and Ramsdell had begun to suspect that a rare human autoimmune disease, IPEX, which is also linked to the X chromosome, might be the human variant of the scurfy mouse’s disease. In 2001, helped by paediatricians from around the world, they found that boys with IPEX did indeed possess harmful mutations in the FOXP3 gene, similar to the mice. These key findings turned out to be decisive in understanding the development of the regulatory T cells discovered by Sakaguchi.
Immune tolerance: how it works
Two years after Brunkow and Ramsdell’s publication in Nature Genetics, Shimon Sakaguchi – and soon other researchers – could convincingly prove that the FOXP3 gene controls the development of regulatory T cells. It was made evident that these cells could prevent T cells from mistakenly attacking the body’s own tissues, an important process of peripheral immune tolerance. Regulatory T cells also ensure the immune system calms down after it has eliminated an invader, so it does not continue working at top speed.
The knowledge researchers gained from the discovery of regulatory T cells and peripheral immune tolerance spurred the development of new treatments to prevent transplant rejection, treat autoimmune diseases and combat tumours, which often hide behind walls of regulatory T cells. The fundamental work initiated in mice has greatly benefitted patients and made a huge impact in the scientific community. There are currently more than 200 clinical trials underway that build on this research. It also laid the foundation for critical discoveries that have revolutionised our understanding of the human body, how it fights infections and how the immune system is regulated and kept in check.
Last edited: 13 October 2025 12:09