Cosmetic testing
-
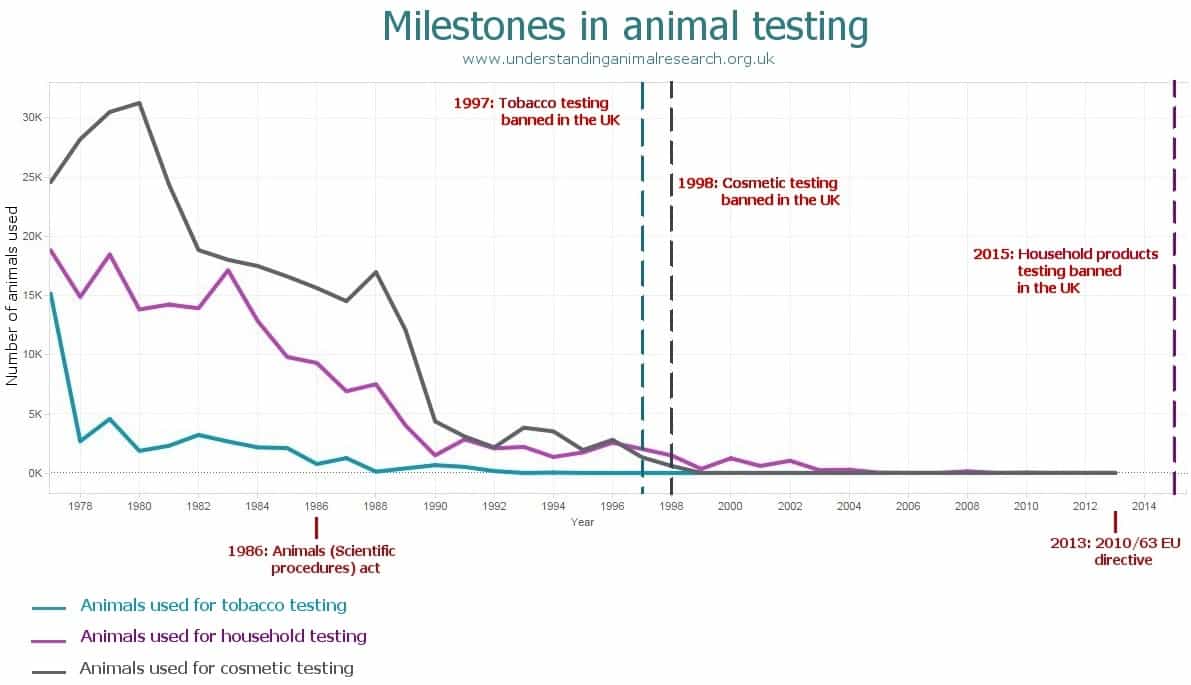
Testing cosmetic products and their ingredients on animals was banned in the UK in 1998 and across the EU in 2013.
-
Recent legal changes have not reintroduced animal testing of cosmetics.
-
EU legislation banning cosmetics experiments is part of EU Regulation 1223/2009 (Cosmetics Regulation).
-
The ban was created because non-animal methods were developed to test the safety of the cosmetics.
Animal testing for cosmetics
In the UK and across the rest of the EU, testing cosmetic products or their ingredients on animals is banned. This means that it is illegal to sell or market a cosmetic product if animal testing has taken place on the finished cosmetic or its ingredients before being sold in the EU.
A ban on animal-tested cosmetic products was first implemented in the UK in 1998 for finished cosmetic products and ‘ingredients intended primarily for “vanity” products’. The EU ban on animal-tested cosmetic products was first passed in 1993 with the full ban taking effect in 2013.
Whilst the UK was a forerunner in banning animal-tested cosmetics this legislation is now part of EU Regulation 1223/2009 (Cosmetics Regulation).
What is a cosmetic product?
The EU defines a cosmetic product as the following1:
“any substance or preparation intended to be placed in contact with the various external parts of the human body (epidermis, hair system, nails, lips and external genital organs) or with the teeth and the mucous membranes of the oral cavity with a view exclusively or mainly to cleaning them, perfuming them, changing their appearance and/or correcting body odours and/or protecting them or keeping them in good condition”
Therefore everyday hygiene products, such as soap, shampoo, deodorant, and toothpaste, as well as luxury beauty items, including perfumes and makeup, are classified as cosmetic products.
Cosmetic products and animal testing
Cosmetic products for sale in the EU (including the UK) must be deemed safe and it is the responsibility of the manufacturer to ensure that they (and their ingredients) undergo scientific safety assessments to prove that they are not toxic to human health.
Before the ban on animal-tested cosmetics was implemented, safety assessments involving the use of animal studies to determine toxicology endpoint were required. The results gathered from these studies measured the effects the cosmetic and its ingredients had on human health and mainly involved the use of rodents and rabbits. When a safety assessment already existed for an ingredient in a new cosmetic product the animal study for the ingredient would not have to be repeated (the animal study for the finished cosmetic would still be required). However, for a new ingredient where a safety assessment did not previously exist, animal studies had to be conducted.
Due to the development of non-animal techniques, it became apparent that these animal studies were no longer required and a ban on animal-tested cosmetics and their ingredients was introduced.
History of the UK ban
In 1998 the Government announced a policy ban to end the use of animal testing for finished cosmetic products and ingredients. The definition of a cosmetic was in line with the concurrent EU description:
“any substance or preparation intended for placing in contact with the various external parts of the human body (epidermis, hair systems, nails, lops and external genital organs) or with the teeth and the mucous membrane of the oral cavity with a view (exclusively or principally) to cleaning them, perfuming them or protecting them in order to keep them in good condition, change their appearance or correct body odours.”
The definition included toothpaste, sun creams and other products which were considered to be pharmaceuticals outside of Europe at the time.
Whilst the ban in the UK was not part of any legislation, the companies involved with animal testing of cosmetic products relinquished their Home Office licences and were not able to renew them.
History of the EU ban
EU Directive 76/768/EEC (Cosmetics Directive) provided the regulatory framework for the phasing out of animal testing for cosmetics purposes across the EU and established a testing ban i.e. it is prohibited to test a finished cosmetic product and its ingredients on animals in the EU, and a marketing ban i.e. it is prohibited to market a finished cosmetic product or its ingredients in the EU if they are tested on animals.
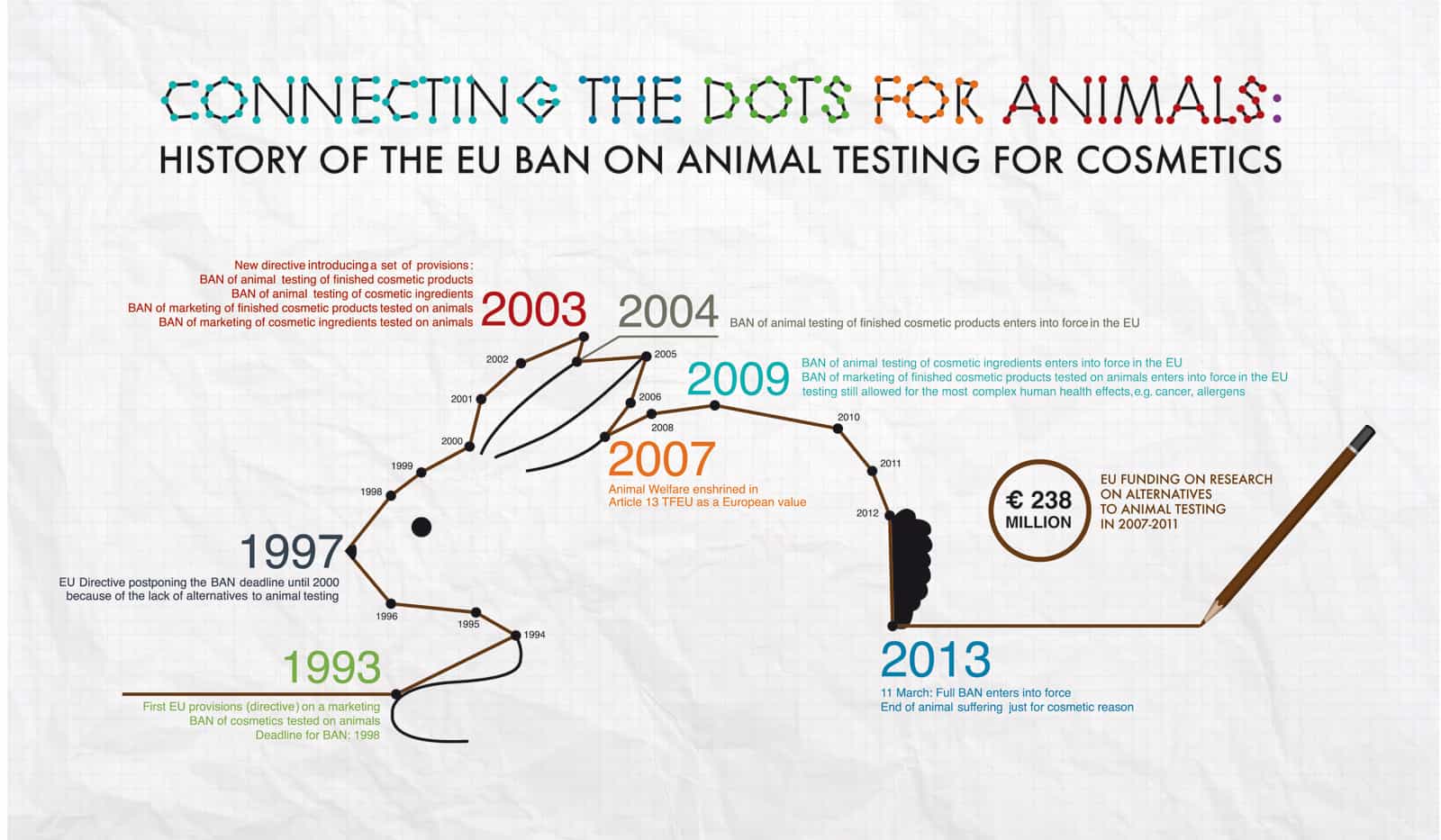
Between 2007 and 2011 the EU spent €238 million on funding replacements, a testament to how serious they are about animal welfare, the 3Rs and the quest to find non-animal methods.
In 1993 the 6th Amendment to EU Directive 76/768/EEC was passed and contained a ban on the sale of animal-tested cosmetic products. To make sure that adequate time was given to finding non-animal methods, the deadline for the ban to come into effect was 1st January 1998.
In 1997 the ban was delayed until 30th June 2000 due to a lack of alternative methods.
In 2000 the ban was delayed until 30th June 2002 due to a lack of alternative methods.
In 2003 the 7th Amendment to EU Directive 76/768/EEC was passed and contained a phased-in ban on animal testing for cosmetics with a deadline of 2013:
Ban animal testing on finished products.
Ban animal testing on cosmetic ingredients.
Ban the marketing of finished products tested on animals.
Ban the marketing of cosmetic ingredients tested on animals.
On 11th September 2004, the ban on animal-tested cosmetic products came into force. The sale of cosmetic ingredients tested on animals outside the EU using methods that have been replaced within the EU was also banned.
On 11th March 2009, the ban on animal testing of cosmetic ingredients within the EU was implemented. The sale of cosmetic products containing newly animal-tested ingredients was banned, however animal testing was still allowed for complex human health issues such as repeat dose toxicity, reproductive toxicity and toxicokinetics.
On 11th March 2013, the full ban came into effect and it is now illegal to market or sell cosmetics in the EU where the finished product or ingredients have been tested on animals.
As of 11th July 2013, EU Directive 76/768/EEC was replaced by EU Regulation 1223/2009 (Cosmetics Regulation) which contains all the same provisions.
Possible exceptions to the EU ban
REACH
REACH is an EU regulation concerning the registration, evaluation, authorisation and restriction of chemicals and is applied to substances manufactured or imported into the EU in quantities of 1 tonne or more per year.
The hazardous properties of chemicals cannot be sufficiently determined using only non-animal methods therefore animal testing is still required to determine certain human health and environmental data. In order to minimise the use of animals REACH requires companies to share data in order to avoid unnecessary testing.
Those wishing to perform animal tests must obtain approval from the European Chemicals Agency (ECHA) which oversees REACH. Animal testing is to be avoided in favour of non-animal methods and registrants can only carry out tests involving the use of animals when there is no other way.
Read more about REACH here.
Substances that are exclusively used in cosmetics
Animal testing is not needed to meet REACH requirements for human health data, with the exception of tests that are done to assess the risks to workers (those involved with the production or handling of the substance on an industrial site) exposed to the substance. In this situation, animal testing is only required when there is no other way.
Animal testing is required to meet REACH requirements for environmental data when there is no other way. Environmental data is used to determine the safety of a chemical in biological organisms and across ecosystems. Environmental studies required for REACH include the use of fish.
In practice, the requirement for an animal test is most likely to come from a new chemical with an unknown safety profile that a cosmetics manufacturer wants to put in a product, or a chemical that is already in use but is now suspected of being dangerous due to new information.
Substances that have mixed-use i.e. not solely used in cosmetics
Animal testing is only used to meet REACH requirements for human health and environmental data when there is no other way.
View this post on Instagram
Cosmetic testing outside the EU
USA
In the USA cosmetics are regulated by the Food, Drug & Cosmetics Act, which prohibits the use of unsafe ingredients in a cosmetic product. The manufacturer has to verify if a cosmetic placed on the market presents any risk to consumer health using methods such as literature reviews, tests on animals or alternative methods, otherwise, the cosmetic must bear the statement ‘the safety of this product has not been determined’. New active ingredients in Over-The-Counter products (anti-acne, anti caries/anti-plaque, anti-hair loss, anti-dandruff, antiperspirant products, skin whiteners, sun protection products) must prove their safety on the basis of animal studies and clinical trials.
Whilst the USA does not prohibit animal testing, the Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM) was set up in 1997 with the aim to reduce animal testing. Once ICCVAM recommends that an alternative method has been adequately validated and the relevant test recommendations are accepted or endorsed by Federal regulatory agencies, the test becomes available for all toxicology purposes.
Japan
A number of products that are qualified as cosmetics in the EU are qualified as quasi-drugs in Japan. This includes hair dyes and de-colourants, anti-hair loss products, hair permanents/straighteners, depilatories, antiperspirants, deodorants, anti-acne, skin whiteners, bath treatment products, and medicinal cosmetics such as anti-dandruff shampoos. These products are subject to the same regulations as pharmaceuticals and a toxicological dossier is required for approval of a new quasi-drug ingredient which includes animal tests when there are no alternatives available.
China
In China, the control of cosmetic products and new cosmetic ingredients is under the responsibility of the Chinese State Food and Drug Administration and local cosmetics regulators. There are different rules for different sorts of cosmetic, and it can matter whether the product was made in China or is to be sold in China.
If, for instance, it is a general cosmetic such as makeup or toothpaste, does not contain a new chemical, is not intended for children and was manufactured in China, then animal testing would not be required. For a general cosmetic that was not manufactured in China, it is possible to avoid animal tests if the manufacturer can prove the product is safe through non-animal methods.
For special cosmetics like hair dyes and sunscreen, animal testing will be required, even if it was manufactured in China, if it is to be sold in China. If it is only to be sold abroad then animal testing is not needed.
References and Links:
- http://ec.europa.eu/growth/sectors/cosmetics/index_en.htm
- EU Regulation 1223/2009 (Cosmetics Regulation)
- RSPCA
- Interface between REACH and Cosmetics regulations
- The EU's role in cosmetics