
Latest figures show a decrease in animals used in research in 2024
-
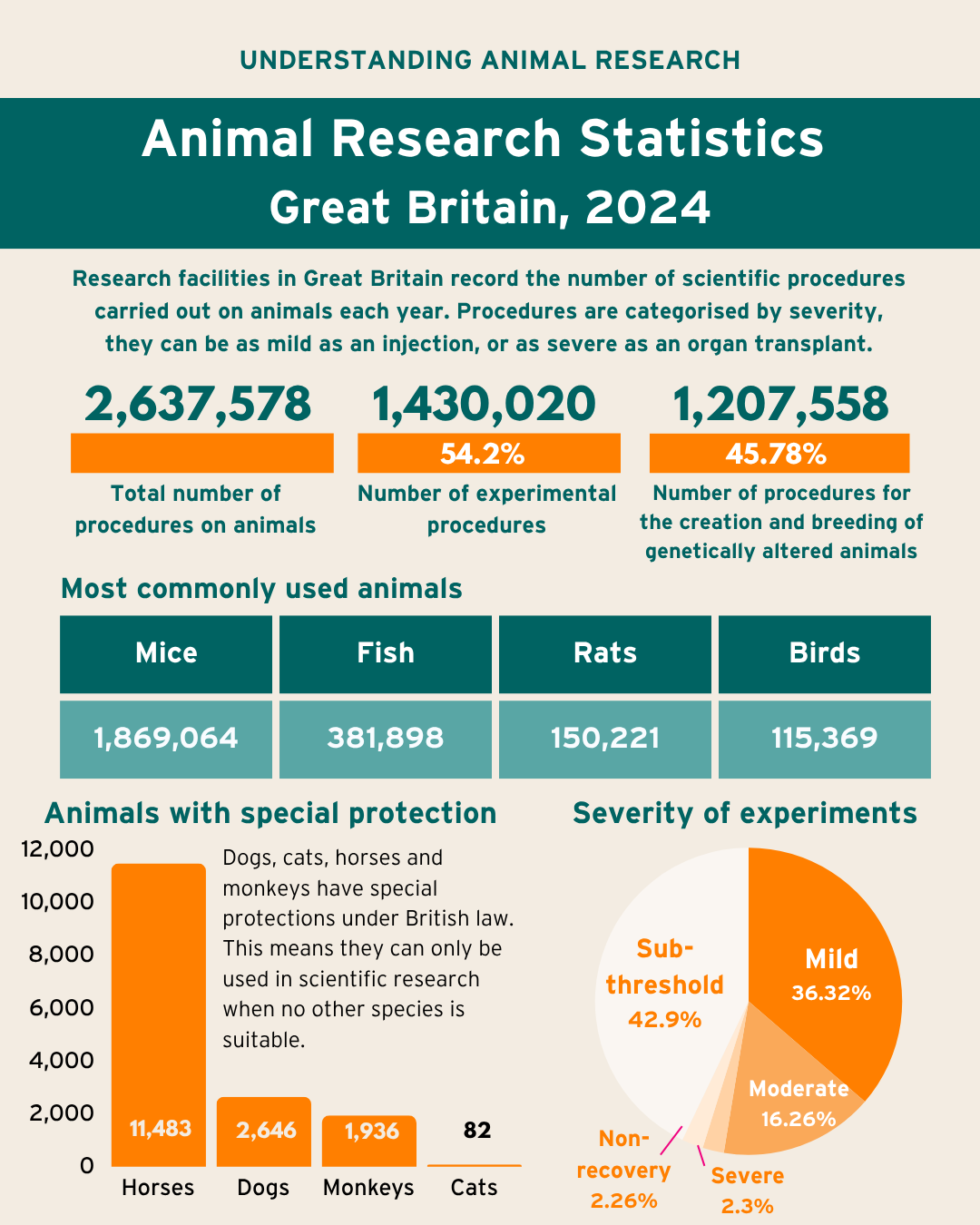
Number of procedures on animals in 2024 has decreased by 1.6% to 2.64 million
-
Lowest number of procedures since 2001
-
Mice, fish, rats, and birds account for 95% of all procedures
-
Cats, dogs, and monkeys account for 0.2% of all procedures
-
Sub-threshold and mild procedures account for 79%
Today (Thursday, 23 October 2025) the government has released its annual statistics on the number of animals used in scientific, medical and veterinary research in 2024. The figures show that 2,637,578 procedures were carried out in Great Britain in 2024, 1.6% less than in 2023.
95% of the procedures were carried out in mice, fish, rats, and birds, whereas cats, dogs, and monkeys accounted for 0.2% of all procedures in 2024.
Download "Animal research statistics Great Britain 2024".
More than half of these procedures were carried out by ten organisations.
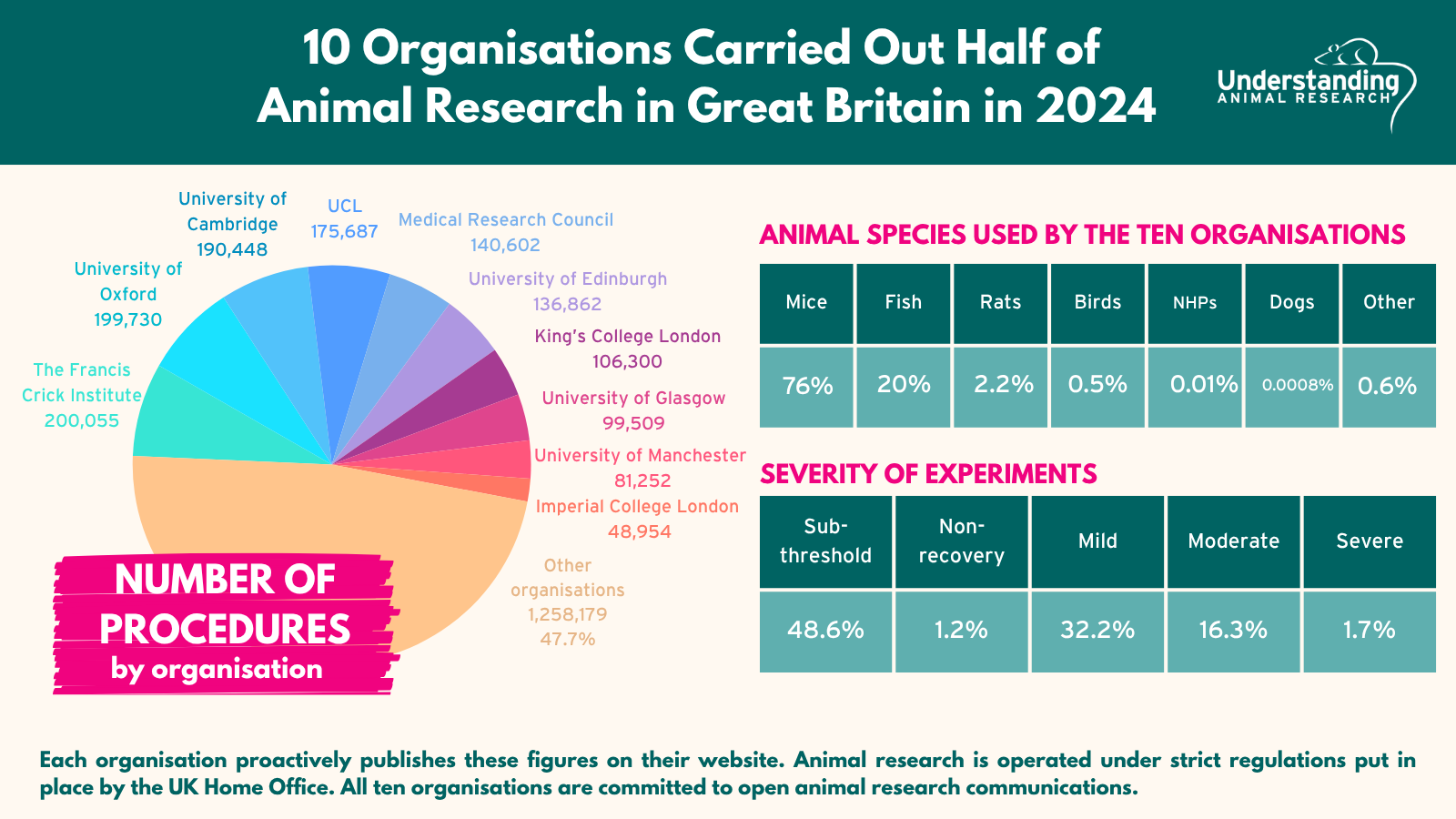
Download "10 organisations carried out half of animal research in Great Britain in 2024"
What is a procedure?
Any procedure applied to a protected animal for an experimental or other scientific purpose, or for an educational purpose, that may have the effect of causing an animal pain, suffering, distress or lasting harm equivalent to, or higher than, that caused by the introduction of a needle in accordance with good veterinary practice.
How many animals are used?
The number of procedures carried out in a year does not equal the number of animals that have been used in procedures that year. This is because some animals may be used more than once i.e. ‘re-used’, in certain circumstances. These instances are counted as separate, additional, procedures. As a result, the number of procedures is usually slightly higher than the number of animals used. 2,554,447 animals were used for the first time in 2024.
Specially protected species
Specially protected species refers to cats, dogs, horses, and non-human primates (monkeys) - they are subject to additional protection under Section 5C of the Animal (Scientific Procedures) Act 1986. This means these species can only be used when no other species is suitable. These species were used in 0.6% of all procedures.
Number of Animal Procedures in Great Britain in 2024
|
Species |
Experimental Procedures |
% |
Procedures for the Creation & Breeding of Genetically Altered Animals |
% |
Total Procedures (2024) |
% |
% change from 2023 |
| Mice | 820,644 | 57.39% | 1,048,420 | 86.82% | 1,869,064 | 70.86% | -2.96% |
| Fish | 229,748 | 16.07% | 152,150 | 12.60% | 381,898 | 14.48% | 3.54% |
| Rats | 145,955 | 10.21% | 4,266 | 0.35% | 150,221 | 5.70% | 1.37% |
| Birds | 114,441 | 8.00% | 928 | 0.08% | 115,369 | 4.37% | -0.08% |
| Other mammals | 90,639 | 6.34% | 53 | 0.00% | 90,692 | 3.44% | 2.70% |
| Reptiles | 38 | 0.00% | 0 | 0.00% | 38 | 0.0014% | 245.45% |
| Amphibians | 12,408 | 0.87% | 1,741 | 0.14% | 14,149 | 0.54% | -18.61% |
| Monkeys | 1,936 | 0.14% | 0 | 0.00% | 1,936 | 0.07% | -10.74% |
| Cats | 82 | 0.01% | 0 | 0.00% | 82 | 0.003% | 30.16% |
| Dogs | 2,646 | 0.19% | 0 | 0.00% | 2,646 | 0.10% | -29.81% |
| Horses | 11,483 | 0.80% | 0 | 0.00% | 11,483 | 0.44% | 0.65% |
| Total | 1,430,020 | 100% | 1,207,558 | 100% | 2,637,578 | 100% | -1.64% |
Purpose of procedures
Experimental procedures and procedures related to the creation and breeding of genetically altered animals decreased by 2.5% and 0.5% respectively, compared to 2023.
Procedures for creation and breeding involve the breeding of animals whose genes have mutated or have been modified. These animals are used to produce genetically altered offspring for use in experimental procedures but are not themselves used in experimental procedures.
46% (1,207,558) of all procedures were for the creation or breeding of genetically altered (GA) animals. Of these 1,207,558 procedures:
-
88% were for the purpose of maintenance of established lines of genetically altered animals
-
12% were for the creation of new lines of genetically altered animals
Experimental procedures involve using animals in scientific studies for purposes such as basic research and the development of treatments, safety testing of pharmaceuticals and other substances, education, specific surgical training and education, environmental research and species protection.
54% (1,430,020) of all procedures were for experimental purposes. This includes basic research, which expands our knowledge of living organisms and the environment; applied research, which addresses the prevention of disease and development of treatments; and regulatory research, which includes studies aimed at ensuring product safety and the effectiveness of pharmaceuticals. Of these 1,430,020 procedures:
-
52% were for basic research
-
24% were for applied research
-
22% were for regulatory research
-
2% were for the protection of the natural environment, preservation of species, and higher education or training
The use of animals to test tobacco products was banned in the UK in 1997 and it has been illegal to use animals to test cosmetic products in this country since 1998. A policy ban on household testing using animals was introduced in 2010. Since 2013, it has been illegal to sell or import cosmetics anywhere in the EU where the finished product or its ingredients have been tested on animals.
The most common research areas for basic research were: nervous system (23%), immune system (18%) and oncology (14%). The most common research areas for applied research were: animal diseases and disorders (32%), human cancer (24%), and human nervous and mental disorders (14%). The most common research areas for regulatory research were: safety testing (50%), routine production of blood-based products (19%), and quality control batch potency testing (17%).
Of the 315,290 regulatory procedures, the most common legislative requirements were legislation on medicinal products for human use (47%) and medicinal products for veterinary use and their residues (27%).
The use of animals to test tobacco products was banned in the UK in 1997 and it has been illegal to use animals to test cosmetic products in this country since 1998. A policy ban on household testing using animals was introduced in 2010. Since 2013, it has been illegal to sell or import cosmetics anywhere in the EU where the finished product or its ingredients have been tested on animals.
Severity of procedures
Severity assessments measure the harm experienced by an animal during a procedure. A procedure can be as mild as an injection, or as severe as an organ transplant.
Severity assessments reflect the peak severity of the entire procedure and are classified into five different categories:
Sub-threshold: When a procedure did not cause suffering above the threshold for regulation, i.e. it was less than the level of pain, suffering, distress or lasting harm that is caused by inserting a hypodermic needle according to good veterinary practice.
Non-recovery: When the entire procedure takes place under general anaesthetic and the animal is humanely killed before waking up.
Mild: Any pain or suffering experienced was only slight or transitory and minor so that the animal returns to its normal state within a short period of time. For example, the equivalent of an injection or having a blood sample taken.
Moderate: The procedure caused a significant and easily detectable disturbance to an animal’s normal state, but this was not life-threatening. For example, surgery is carried out under general anaesthesia followed by painkillers during recovery.
Severe: The procedure caused a major departure from the animal’s usual state of health and well-being. This would usually include long-term disease processes where assistance with normal activities such as feeding and drinking were required, or where significant deficits in behaviours/activities persist. Animals found dead are commonly classified as severe as pre-mortality suffering often cannot be assessed. Most severe procedures arise in regulatory testing such as the evaluation of the toxicity of drugs.
Sub-threshold and mild procedures account for 79% of all procedures in 2024.
Severity of Animal Procedures in Great Britain in 2024
|
Severity |
Experimental Procedures |
% |
Procedures for the Creation & Breeding of Genetically Altered Animals |
% |
Total Procedures (2024) |
% |
Change from 2023 (based on procedure numbers) |
| Sub threshold | 229,020 | 16.02% | 902,514 | 74.74% | 1,131,534 | 42.90% | 3.13% |
| Non-recovery | 58,367 | 4.08% | 1,334 | 0.11% | 59,701 | 2.26% | -7.64% |
| Mild | 692,495 | 48.43% | 265,593 | 21.99% | 958,088 | 36.32% | -6.46% |
| Moderate | 401,914 | 28.11% | 27,065 | 2.24% | 428,979 | 16.26% | -1.28% |
| Severe | 48,224 | 3.37% | 11,052 | 0.92% | 59,276 | 2.25% | -3.03% |
| Total | 1,430,020 | 1,207,558 | 2,637,578 |
Why has the total number of procedures decreased this year?
The total number of animals used in research is affected by many factors. The overall funding for life sciences in the United Kingdom, as well as the relative funding in other countries, will change the amount of science done – a proportion of which will involve animals.
Animals are used alongside other techniques such as cell cultures, human studies and computational models. These methods are used – often in tandem – to answer the key biological questions necessary to understand and treat disease.
Animal research is strictly regulated in the UK. Every procedure, from a simple blood test to major surgery, requires individual, establishment and project licences, as well as approval from an Animal Welfare and Ethical Review Body. Before an animal is used, researchers must show that the knowledge could not be acquired using non-animal methods.
While the government produces these statistics on an annual basis, more organisations than ever before are openly publishing their own figures on their websites. This move towards greater transparency has been bolstered by the Concordat on Openness on Animal Research in the UK, which has been signed by more than 130 organisations since it launched in 2014.
Chris Magee, Head of Policy and Media, Understanding Animal Research, said:
"Animals are used in research in the UK to help discover, develop and test new medicines for humans and animals. They are also used for conservation research and to protect our environment and the safety of our food. No animal is used if there’s an alternative, and no animals are used to test cosmetics, tobacco, alcohol or household products. The number of animals used each year can go up or down depending on various factors, including investment in the UK biosciences.
"Overall numbers have been on a gradually decreasing trend since the mid-1970s, and 2024’s drop is in line with that trend.
"The 2024 statistics seem much as in previous years. Just under half of procedures are breeding animals with particular genetic qualities, and the rest are more traditional categories, such as medical research. Procedures using mice, fish, rats and birds are the most common by far, making up 95% of the total. Experiments which result in severe suffering continue to be relatively rare at 2.25% of all procedures (3.27% of experimental procedures and 0.92% of breeding procedures).
"The number of dogs used decreased by about a third. There is currently no alternative to using dogs for some regulatory toxicology requirements, so it is unclear at this time whether this is due to work moving abroad, and outside of the special protections for dogs in research, which are unique to our country, or whether fewer potential new medicines went through toxicology testing in 2024.
"The number of amphibians used in research tripled in 2023, and we see similar numbers persisting into 2024, although the focus of the research has shifted from translating findings into humans back towards basic research into how biology functions.
"We will always avoid animal use where we can, but these figures show that in a wide range of areas animal research remains the only proven way of saving and improving human and animal lives."
Last edited: 24 November 2025 10:27



