Common myths around animal research
1. “92% of animal-tested drugs fail”
86% of drugs fail to get to market, but it has little to do with animal experiments.
Animals have a limited role in drug testing. They are used to see if a drug is safe enough to try in humans and they predict this with 86% accuracy on average but with most values above 90%. The drug may then fail for other reasons like not being effective or commercial reasons. Success rates vary by area – 33.4% of infectious-disease vaccines are approved, whereas the average for cancer drugs is 8.3%.
2. “Animal experiments have never been validated as working”
Validation simply means proving that a research method works, and it comes in several forms. Animal tests are validated by sound study design and looking back at decades of data, whereas new methods don’t have 150 years of information to draw from. Thus, they must prove their effectiveness through specially-commissioned tests. In 1969, McKinney and Bunney were the first to propose criteria on the external validity of animal models, mainly focused on affective disorders. In 1984, Wilner simplified these external validations to three: predictive, face, and construct validity. These remain the most widely-accepted criteria for external animal model validation. However, internal validity (experimental design, reproducibility, randomization, control, etc.), applies to all scientific research and the precedent for using a particular animal model for a particular application is also contained in the scientific literature specific to that application e.g. immunology.
3. People are killed as a result of animal tests
No drug is licensed just on animal tests – the drug will mainly have been tested on non-animal assays and humans. In UK drug testing trials there have been no deaths and only one serious incident since 1968. In that incident, in 2006, none of the participants died. The only death in living memory during phase 1 human trials was in France in 2016, where 90 volunteers were given a drug, six fell ill and one died. Just 0.3% of phase 1 clinical trials result in a serious adverse event.
4. Animals are used when superior, non-animal methods could be used instead.
Animal tests are only allowed by law when an alternative cannot be used. New non-animal methods are being developed that can equal or outperform animal tests, but for tasks animals are generally not used for. An example is the Emulate human liver chip, which recently was found to predict both short and long-term liver danger with 87% accuracy. At present, animals are used to predict short-term safety with 87% accuracy. Using the liver chip could prevent drugs advancing to regulatory approval if it returns a positive result, but animals will still be needed to ensure the safety of all the other organs.
5. Penicillin was delayed by a decade because of animal tests
Penicillin was difficult to purify and various chemists spent a decade trying and failing. Once purified, the next issue was to make enough to treat even a single person. New industrial processes had to be developed to scale up production to the point that lives could be saved. The full story with references is here.
 6. It’s all about Money
6. It’s all about Money
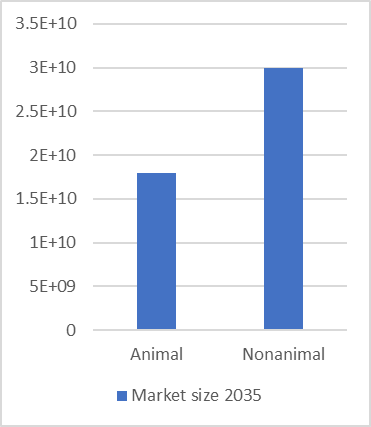
The market for animal and non-animal methods are roughly the same size. The market for non-animal methods is expected to grow at a rate of 13.5% to 2035, to £30 Billion, whereas the market for animals will grow by around 5% to around £18 Billion.
Most animal research is undertaken by public bodies, not private entities, and regulations are set by regulators that can’t make money. The accusation that things are ‘all for money’ is very common across lots of areas of science denial. Climate change deniers accuse climatologists of it, anti-vaxxers accuse pharma companies (or Bill Gates) of it.
7. Animals are tortured
Torture has the specific meaning of inflicting severe pain with the sole intention of causing pain. Severe suffering is rarely intentional. Around 2.5% of experiments are in the ‘severe’ category. This can mean severe suffering, extended moderate suffering, unexpected death or a severe departure form a normal state. The greatest single area where severe suffering occurs in safety testing bathes of potentially dangerous products.
Some species, like dogs and cats, are very unlikely to be involved in severe experiments whereas fish are much more likely to experience severe suffering because they are used for things like ecotoxicity, where we need to understand what happens when something enters the natural environment.
8. It’s all about pharmaceuticals
Animal research is mainly basic research. Pharmaceuticals are safety tested on animals but this is only 10% or less of experiments.
9. Cosmetics and household cleaners are tested on animals
Cosmetics tests were banned in 1998 and household testing was banned in 2025.



