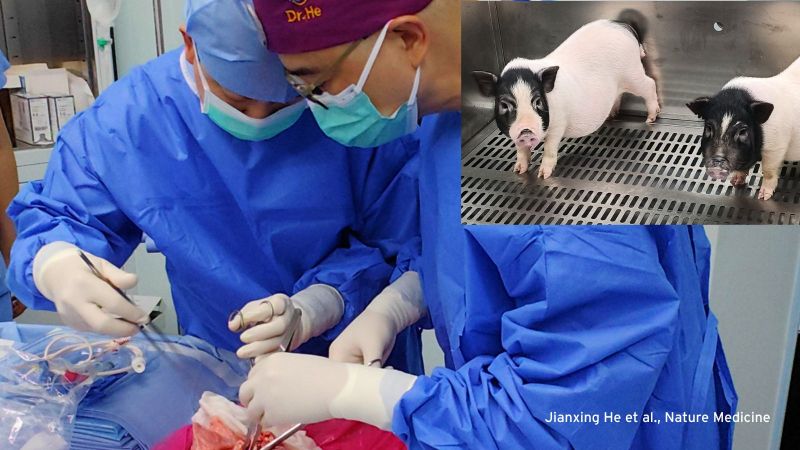
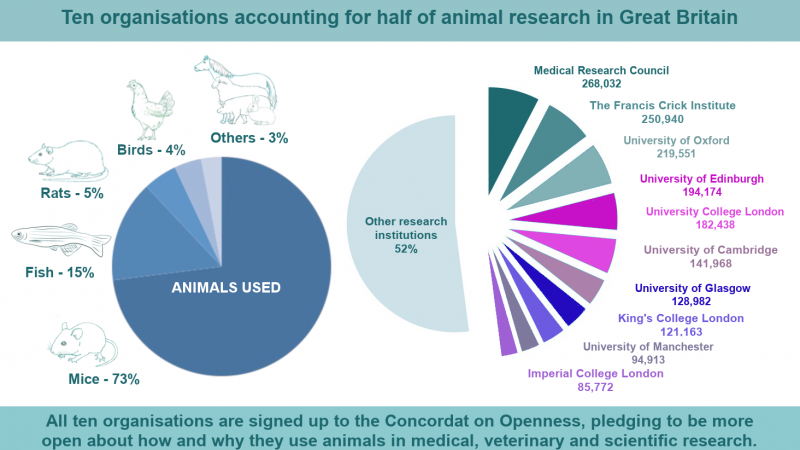
On 7 January 2022, for the first time ever, a pig heart was transplanted into a human.
With over 10,000 people in the UK currently in need of a new organ, this operation provides hope that there may be a way to overcome the chronic shortage of donor human organs.
“This is a great step forward – you can compare it with the first landing on the moon,” said Joachim Denner from the Free University of Berlin to New Scientist. But like space exploration, it didn’t happen overnight. Rather, it relied on decades of prior research. For the medical team who carried out the operation, it marks the culmination of years of work, and it will probably take many more similar transplants before the procedure becomes widely available – if it ever does.
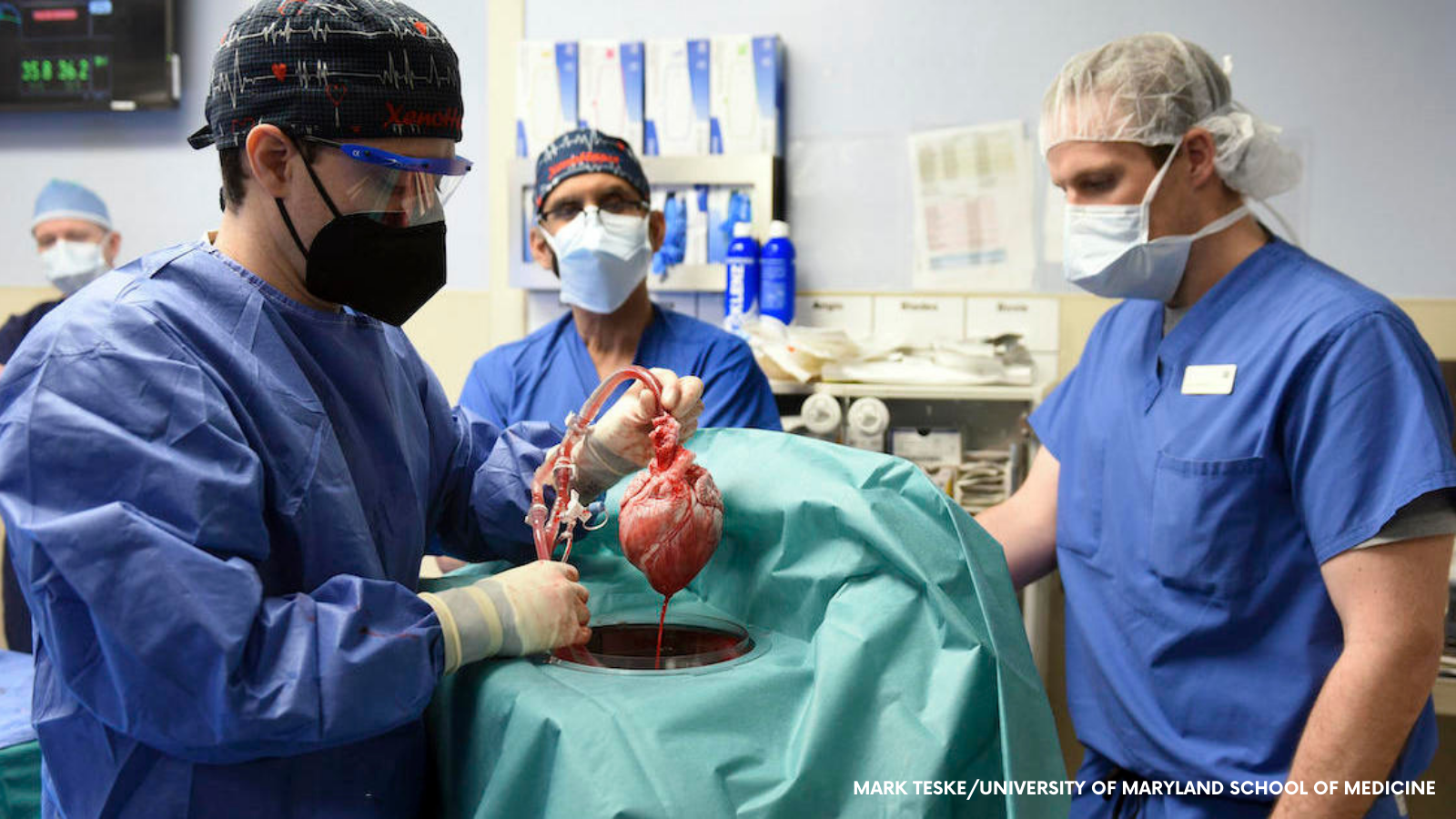
Xenotransplantations – animal to human transplants – are technically demanding on many levels. Surgeons have been attempting to put baboon and chimpanzee organs into humans since at least the 1960s. In 1984, a human infant even received a heart from a baboon but she died 21 days after the transplant.
Primates fell out of favour in the 1990s as potential organ donors because the risk of viral infections was deemed too great.. Not just any heart from any animal can be harvested and transplanted into humans, or it would have been done decades ago. The organ has to be a similar size to that of a human and that is what brought pigs into the research spotlight.
Tackling organ rejection
Pig hearts are anatomically and functionally similar to human hearts but, obviously not identical. These differences can cause organ rejection. This happens when the body's immune system treats a new organ as an unwelcome a foreign object and attacks it. This also happens in human-to-human transplants, even between carefully immunologically matched human donors and recipients.
After unfruitful trials with transplanted organs from human cadavers, many research groups in the 60s turned to animals. They wanted to find a way to modify animals so their organs provoked less of an immune reaction in humans.
Following work in rabbits, guinea pigs and rats, a first group succeeded in producing a biologically inert, functional and durable heart valve in the 1970s. After they were tested in dogs, these xenografts, usually from pigs, were successfully used in humans. To this day, these ‘bioprosthetic’ valves are used to treat some 60,000 patients per year in the USA. Tissue valves are generally preferred over mechanical ones and can last for about 15 years.
This was a great advance but, unfortunately, the bio prosthesis necessitated washing, denaturing and tanning processes that rendered the tissue inert. This meant that it couldn’t be applied to whole-organ transplants. For this, the whole tissue needed to be kept alive and functional.
A solution to the rejection problem began to emerge in the 1990s with the increased understanding of the immune system. In 1993, surgeon David Cooper of the University of Pittsburgh and his colleagues discovered that most of the human immune reaction was directed at a single pig antigen: a sugar molecule called α-1,3-galactose. Present on the surface of cells, it can cause organ rejection within minutes. Suppressing its production tempered the immune reaction, but unfortunately not enough. Baboons that received pig organs never survived longer than a few weeks, even when treated with a combination of different immunosuppressant drugs.
The immune system turned out to be much more complex than anticipated. Different organs posed different problems. Kidneys tended to be safer to transplant than hearts, while lungs were extremely difficult to transplant because of their extensive networks of blood vessels, which provide more opportunities for primate blood to meet pig proteins and coagulate.

Understanding the pig genome
The second part of the puzzle came with a better understanding of the pig genome.
In 2003, David Ayares and his colleagues created the first cloned pig genetically modified to delete α-gal. These modifications greatly increased the time a pig organ could survive in a baboon. The primates could live up to 2.5 years with a functional heart from an α-gal-free pig beating in their abdomen alongside their own . The pig had also received two human genes that protect from coagulation.
2012 was a turning point in animal-human hybrid engineering. It was not only the year the pig genome project was released – the first annotated sequenced pig genome, following the successful generation of genetic and physical maps for the pig – but also the year the genetic scissors CRISPR-Cas9 system was invented.
Armed with this newfound knowledge of the genome, and the recent genetic technological breakthroughs, scientists started to engineer safer animal organs for human transplants, much more quickly, precisely and accurately than it had been possible to do in the past.
“Our first [α-]gal-knockout pig took three full years,” Joseph Tector, a transplant surgeon at Indiana University in Indianapolis told Nature. “Now we can make a new pig from scratch in 150 days.” His group used CRISPR to knock out two pig genes simultaneously in 2015. Macaques transplanted with these pig organs survived for more than three months.
In 2015, Cooper also announced that a kidney transplant from a genetically modified pig with six modified genes supported a baboon for 136 days. In 2016, a transplanted heart from a genetically modified pig survived 51 days in a monkey. From then on, scientists just got closer and closer to their goal of transplanting a pig heart into a human.
But the research didn’t always go in a straight line.
Looking for the answer in stem cells
One of the major concerns in using pig tissues in humans was that virus genes naturally found in pig DNA could cross over into humans. The pig genome contains 62 copies of porcine endogenous retroviruses. To prevent this, researchers created pig cells using CRISPR-Cas9 that lacked these retroviruses. After several trials, all 62 occurrences of the PERV genes were inactivated in pig kidney cells.
Another potential solution was to grow human organs in pigs that could then be transplanted back into humans. In parallel to genetic engineering, researchers also explored the use of stem cells to grow human organs in animals.
In 2016, a team of scientists from the University of California injected human stem cells into pig embryos to produce chimeric embryos. The animals that develop from these embryos look and behave like normal pigs except that their pancreas – or any other organ of interest – would be composed of human cells.
The creation of these chimeric embryos happens in two stages. First, DNA that enables the foetus to grow a pancreas is removed using CRISPR-Cas9. This creates a developmental void. Without this void, previous experiments had shown that human cells struggle to compete with the pig cells and develop inside the pig.
Then, human induced pluripotent (iPS) stem cells are injected into the embryo. The iPS cells are derived from adult cells, possibly from a specific patient, and dialled back to become stem cells capable of developing into any tissue in the body. In theory, the resulting organs would be an exact genetic copy of the patient’s but a much younger and healthier version and would not need the prescription of immunosuppressive drugs which carry side-effects.
The first steps in xenotransplantation into humans – pig corneas
The first time live animal tissue was successfully transplanted into humans, it was in the form of a few cells – a much simpler goal than using whole organs because the immune response is not as severe.
In 2016, Chinese regulators approved the use of pig corneas to restore sight in humans after corneal disease. Over 200 patients underwent the procedure and recovered their eyesight.
That year, researchers also started studying how to transplant insulin-producing islet cells from pigs to humans. This could eliminate the need for insulin shots for patients with diabetes. Bernhard Hering, a transplant surgeon at the University of Minnesota in Minneapolis, and his group used CRISPR to create pig pancreatic islets that could be transplanted without the need for immunosuppressive drugs.
Next, in 2019, experts from the US announced that live-cell, genetically engineered pig skin could temporarily close a burn wound.
With these advances, the fears that virus genes naturally found in pig DNA could cross to humans faded. No such problems arose when pig pancreatic cells were transplanted in humans nor with transplants of whole pig organs into primates.
From cells to organs
The challenge of using whole organs was met only last year, when a brain-dead person with no hope of recovery received a genetically-altered pig kidney. At the time, the operation was the most advanced experiment in the field. The transplanted kidney functioned well and paved the way for the next – and final – step.
Finally, after all this extensive work, David Bennett, 57, received on 7t January 2022, in a last-hope operation, a heart from a pig that had been genetically modified to boost the chances of acceptance in a human body.
To bypass rejection, the animal had 10 genes modified. Four of those were inactivated, including one that causes an aggressive immune response and one that would otherwise cause the pig’s heart to continue growing after transplant into a human body.
To further increase the chances of acceptance, the donor pig had six human genes inserted into its genome and Bennett is taking immune-suppressing medications, slightly different than for a normal human-to-human transplant. These first days are a critical test, although immune rejection could take weeks or longer to develop.
A spokesperson for NHS Blood and Transplant in the UK said in a statement: “We have been watching this particular field of research for many years. However, there is still some way to go before transplants of this kind become an everyday reality.” Although giving people animal organs is not ideal compared to swapping in a human donor heart, it could save many lives where human organs are unavailable.
After two months, Mr Bennett passed away on March 8th, 2022
The first-ever recipient of a pig-heart transplant died two months after his surgery. While it is not clear what the exact cause of Mr Bennett's death is, this experimental surgery always presented a risk and was only approved as a final treatment option. Further investigations will determine the next steps in this critical research to solve the global shortage of transplant organs.
Last edited: 4 August 2022 16:27