Latest figures show fewer animals used in research in 2020
-
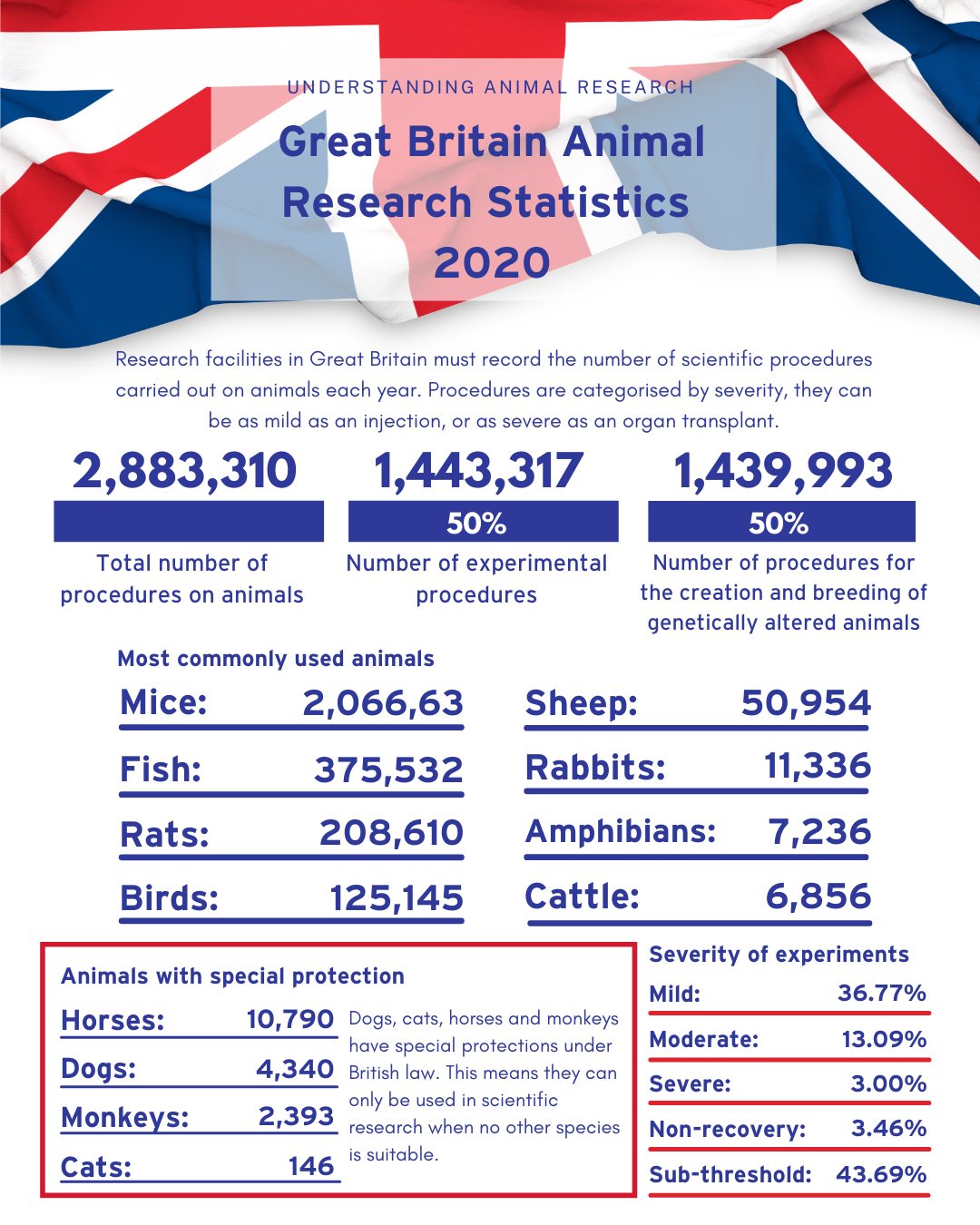
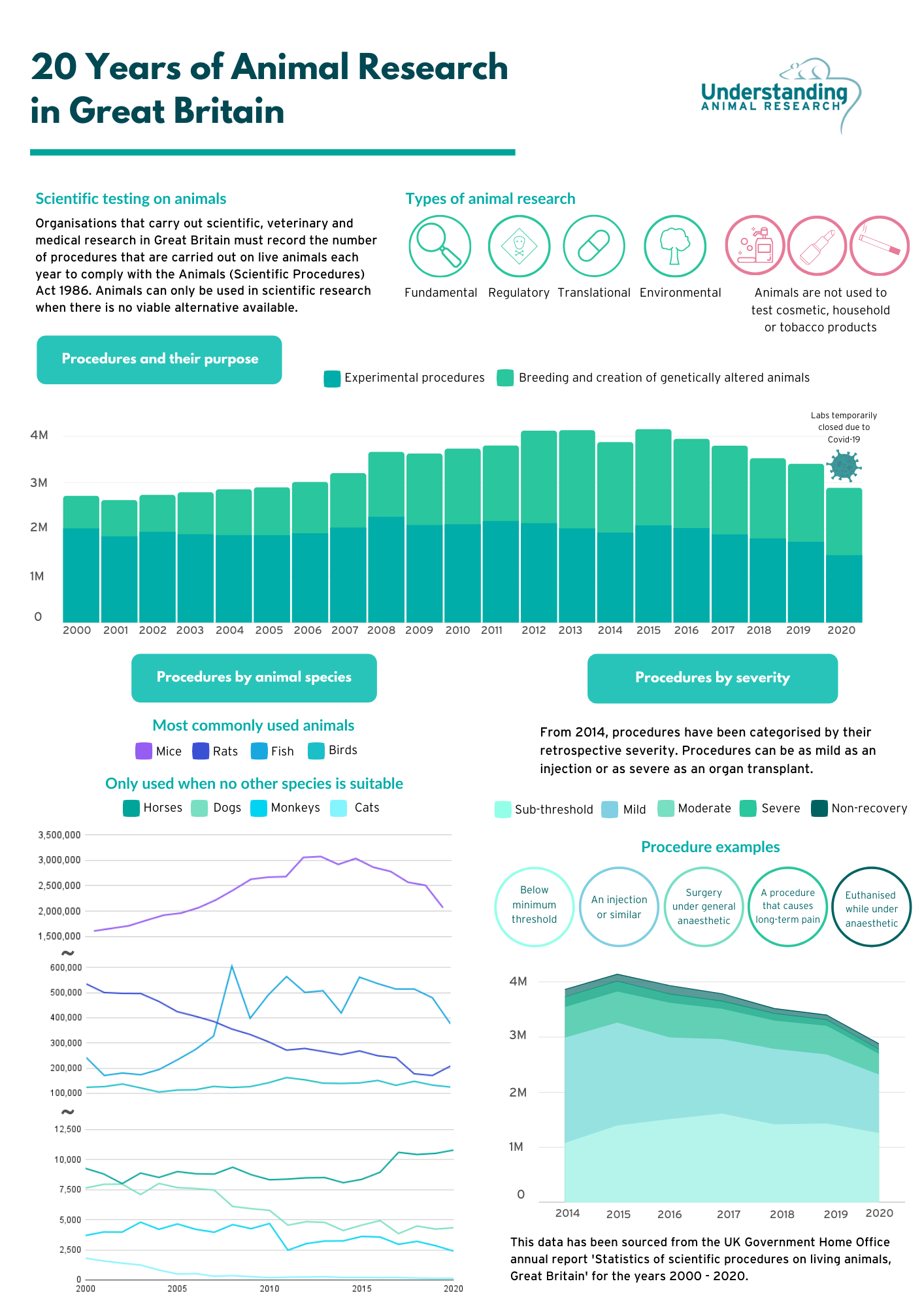
Number of procedures on animals in 2020 has decreased by 15% to 2.88 million
-
Large decrease in numbers due to various Covid-19 national lockdowns
-
Lowest number of procedures since 2004
-
Mice, fish, rats, and birds account for over 96% of all procedures
-
Cats, dogs, and primates account for 0.2% of all procedures
Today (Thursday, 15 July 2021) the government has released its annual statistics on the number of animals used in scientific, medical and veterinary research in 2020. The figures show that 2,883,310 procedures were carried out in Great Britain in 2020, 15% fewer than in 2019.
Animal research and COVID-19
In 2020, animal research has been essential for developing lifesaving vaccines and treatments for Covid-19. Ferrets and macaque monkeys were used to test the safety and efficacy of Covid-19 vaccines, including the successful Oxford-AstraZeneca vaccine. Hamsters are being used to develop Covid-19 treatment strategies as they display a more severe form of the disease than ferrets and monkeys. Guinea pigs have also been used in regulatory research to batch-test vaccine potency.
Despite all this research to develop vaccines and treatments for Covid-19, the majority of UK research facilities carried out significantly less research than usual, due to the various national lockdowns. Therefore, the 2020 figures cannot be reasonably compared with previous statistics.
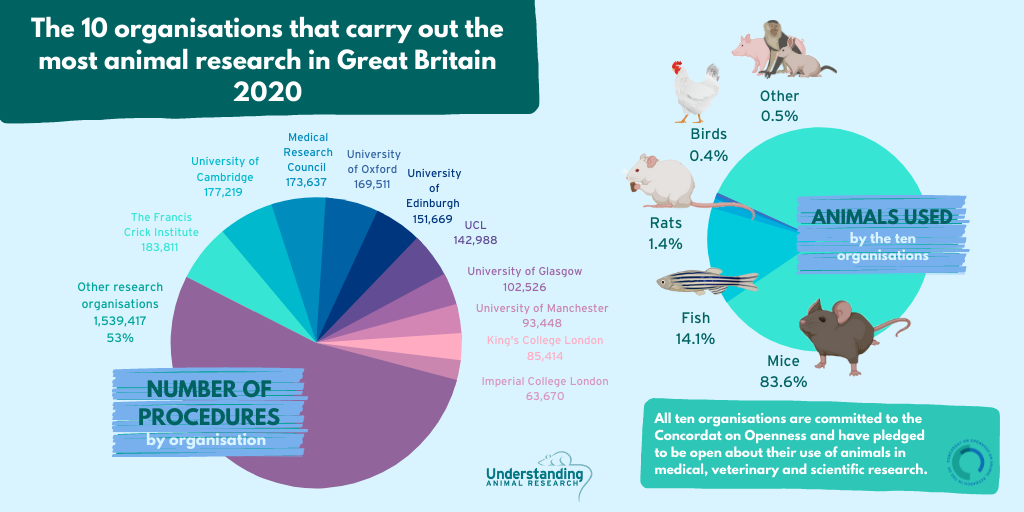
Nearly half of these procedures were carried out by ten organisations.
Over 96% of procedures were carried out on mice, fish, rats and birds whereas cats, dogs and primates accounted for 0.2% of all procedures.
Number of Animal Procedures in Great Britain in 2020
| Species | Experimental Procedures | % | Procedures for the Creation & Breeding of Genetically Altered Animals | % | Total Procedures (2020) | % | % change from 2019 |
| Mice | 827,801 | 57.35% | 1,238,834 | 86.03% | 2,066,635 | 71.68% | -17.58% |
| Fish | 189,200 | 13.11% | 186,332 | 12.94% | 375,532 | 13.02% | -21.70% |
| Rats | 198,763 | 13.77% | 9,847 | 0.68% | 208,610 | 7.24% | 21.94% |
| Birds | 123,157 | 8.53% | 1,988 | 0.14% | 125,145 | 4.34% | -5.77% |
| Other mammals | 82,328 | 5.70% | 155 | 0.01% | 82,483 | 2.86% | -3.43% |
| Reptiles | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 0% |
| Amphibians | 4,399 | 0.30% | 2,837 | 0.20% | 7,236 | 0.25% | -1.90% |
| Primates | 2,393 | 0.17% | 0 | 0.00% | 2,393 | 0.08% | -16.04% |
| Cats | 146 | 0.01% | 0 | 0.00% | 146 | 0.01% | 11.45% |
| Dogs | 4,340 | 0.30% | 0 | 0.00% | 4,340 | 0.15% | 2.67% |
| Horses | 10,790 | 0.75% | 0 | 0.00% | 10,790 | 0.37% | 2.64% |
| Total | 1,443,317 | 50.06% | 1,439,993 | 49.94% | 2,883,310 | 100% | -15.23% |
Half (1,439,993) of all procedures were for the creation or breeding of genetically altered (GA) animals. Of these 1,439,993 procedures, 90% (1,293,114 procedures) were for the purpose of maintenance of established lines of genetically altered animals (not used in experimental procedures), while 10% (146,879 procedures) were for the creation of new lines of genetically altered animals (not used in experimental procedures).
The other half (1,443,317) of all procedures were for experimental purposes. This includes basic research, which expands our knowledge of living organisms and the environment; applied research, which addresses the prevention of disease and development of treatments; and regulatory research, which includes studies aimed at ensuring product safety and the effectiveness of pharmaceuticals. Of these 1,443,317 procedures, 52.5% (758,091 procedures) were for basic research, 32.8% (473,083 procedures) were for regulatory research, and 13.6% (195,697 procedures) were for applied research.
The use of animals to test tobacco products was banned in the UK in 1997 and it has been illegal to use animals to test cosmetic products in this country since 1998. A policy ban on household product testing using animals was introduced in 2010. Since 2013, it has been illegal to sell or import cosmetics anywhere in the EU where the finished product or its ingredients have been tested on animals.
Severity of Animal Procedures in Great Britain in 2020
| Severity | Experimental Procedures | % | Procedures for the Creation & Breeding of Genetically Altered Animals | % | Total Procedures (2020) | % | Change for 2019 |
| Sub threshold | 207,471 | 14.37% | 1,052,212 | 73.07% | 1,259,683 | 43.69% | ↓ |
| Non-recovery | 98,456 | 6.82% | 1,187 | 0.08% | 99,643 | 3.46% | ↑ |
| Mild | 729,655 | 50.55% | 330,577 | 22.96% | 1,060,232 | 36.77% | ↓ |
| Moderate | 349,236 | 24.20% | 28,121 | 1.95% | 377,357 | 13.09% | ↓ |
| Severe | 58,499 | 4.05% | 27,896 | 1.94% | 86,395 | 3.00% | ↓ |
| Total | 1,443,317 | 1,439,993 | 2,883,310 |
Severity assessments measure the harm experienced by an animal during a procedure. A procedure can be as mild as an injection, or as severe as an organ transplant, and the severity assessments reflect the peak severity of the entire procedure.
Sub-threshold is when a procedure did not cause suffering above the threshold for regulation. Non-recovery is when the entire procedure takes place under general anaesthetic and there is no recovery. Mild is the equivalent of an injection or having a blood sample taken. Moderate includes surgery under anaesthesia followed by painkillers during recovery. Severe suffering is when a procedure causes a major departure from the animal's usual state of health and well-being. Animals found dead are commonly classified as severe as pre-mortality suffering often cannot be assessed. Most severe procedures arise in regulatory testing such as evaluation of toxicity of drugs.
The proportion of animal research categorised as sub-threshold increased from 42.11% in 2019 to 43.69% in 2020. Mild procedures decreased from 36.82% to 36.77%, moderate procedures decreased from 15.42% to 13.09%, and severe procedures decreased from 3.11% to 3.00%. The proportion of animal research categorised as non-recovery rose from 2.53% in 2019 to 3.46% in 2020.
Animals are used alongside other techniques such as cell cultures, human studies and computational models. These methods are used – often in tandem – to answer the key biological questions necessary to understand and treat disease.
Animal research is strictly regulated in the UK. Every procedure, from a simple blood test to major surgery, requires individual, establishment and project licences, as well as approval from an Animal Welfare and Ethical Review Body. Before an animal is used, researchers must show that the knowledge could not be acquired using non-animal methods.
While the government produces these statistics on an annual basis, more organisations than ever before are openly publishing their own figures on their websites. This move towards greater transparency has been bolstered by the Concordat on Openness on Animal Research in the UK, which has been signed by 128 organisations since it launched in 2014.
Wendy Jarrett, Chief Executive, Understanding Animal Research (UAR), said:
“Animal research has been essential to the development and safety testing of lifesaving COVID-19 vaccines and treatments. Macaque monkeys and ferrets have been used to develop vaccines, including the Oxford-AstraZeneca vaccine, hamsters are being used to develop treatments, and guinea pigs are used to quality-check each batch of vaccines. Animal testing provided scientists with initial data that the vaccines were effective and safe enough to move into human clinical trials. During these trials, thousands more humans than animals were used to test how effective and safe the vaccines were in people. The pandemic has led to increased public interest in the way vaccines and medicines are developed and UAR has worked with research institutions and funding bodies throughout the UK to develop resources that explain to the public how animals have been used in this critical research.”
Last edited: 1 November 2022 11:23




