The recent announcement of a potential malaria vaccine that is 77% effective in early trials is a reminder that animal research remains an essential weapon in the battle against human disease.
It is caused by a plasmodium parasite that grows and evolves fast, and takes different forms depending on whether it infests the liver or blood cells.
A vaccine has to trigger the body into producing an effective immune reaction against this complicated foe.
Growing the parasite outside of a human body allows us to safely test different potential vaccines.
This is where animal research becomes essential.
Historically, researchers used birds to study the malaria parasite and develop medications, such as chloroquine. Better knowledge of the parasites led to the discovery of new drugs and sent researchers on a vaccine quest. For example, antibodies created to fight the blood stages of infection could be transferred from one person to another, conferring some protection. But malaria is such a complex parasite that it proved harder to defeat than many had first hoped.
“Unfortunately, the energy put into the vaccine research didn’t live up to expectations. The field didn’t move nearly as fast as I believed it could,” says Jacob Baum, Professor of Cell Biology and Infectious Diseases at Imperial College London. But he is enthusiastic about the future: “If some of the energy and breakthroughs are harnessed from the COVID crisis and put into malaria research, I believe there are strategies that could really transform the landscape. With a child dying every minute or two, half a million deaths a year, malaria has a bigger impact than the new coronavirus. The parasites have killed far more people than any other infectious disease.”
Today, in vitro models, monkeys and rodents play a key role in the discovery and development of anti-malarial drugs and vaccines. Advances in animal research have enabled scientists to model human malarial symptoms much more closely in mice.
“Thanks to remarkable work in mass genetics, it is now also possible to use humanised mouse models. In those immune-deficient models, you can generate a chimeric liver, which is a human liver sort of transplanted onto the mouse liver, and then the human parasites will be able to infect that liver,” explains Prof Baum.
“More recently, in the last five years or so, there has been a push to develop an entirely invitro liver platform. But we are not quite there yet. We haven’t quite got full liver development all the way through to the parasites being able to immerge and then go into blood cells culture, although that’s on the horizon. I think it’s only about five or 10 years away,” he adds.
There is also something unique about malaria research: humans - under very strictly regulated conditions - can be infected with malaria experimentally because it is possible to cure them. This means that a human model of the disease can be used in some cases instead of animals, which is an extremely powerful tool, especially when trying to find a vaccine.
“A lot of research is moving in that direction. So if you have an experimental strategy and you can show it’s safe you can actually quite quickly move to human trials, having done the groundwork in rodent malaria models. But there are obvious limitations,” Prof Baum explains.
“The rodent malaria model has been absolutely essential for research. In order to develop a new vaccine candidate or target you have to test it in a living, breathing system. All of our understanding of the biology of the liver stages, how the parasite gets from the skin to the liver or it doesn’t, how it interacts with the immune system, how it emerges from the liver, that’s all been entirely dependent on the rodent malaria model. There are huge areas of our understanding that couldn’t have been filled without the rodent malaria model,” concludes Prof Baum.
This animal research and the years of accumulated knowledge that has stemmed from it have fed into the success of the vaccine that recently made the news. The vaccine is a low-dose protein-based vaccine (R21) that targets the first part of the malarial parasite life cycle in the human, the sporozoite, by aiming at a protein secreted by the sporozoite called Circumsporoite .
If the next stage of the trial works as well as the first, we might at last see an effective vaccine against Malaria.
Reference
https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3830681
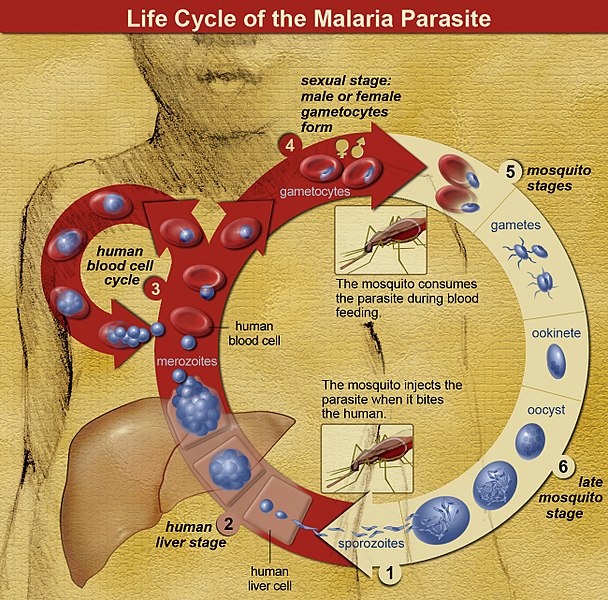
Malaria is caused by a plasmodium parasite. The parasite spreads to humans and other animals through the bites of infected female mosquitoes. As the plasmodium spreads and reproduces in the body it causes symptoms include fever, tiredness, vomiting, and headaches. In severe cases, the parasites can cause yellow skin, seizures, coma, or death.
There are more than 200 species of parasites in the Plasmodium genus. Each species has a different life cycle and history. Humans are infected by only five of these, but reptiles, birds and other mammals can also fall sick to the disease.
The life cycle of the parasite was discovered in the 1880s by Dr Alphonse Laveran and with it, the role of female mosquitos in the spread of the disease. Disease transmission mostly depends on how well the mosquitos fare. It is more intense where the climate is favourable to the insects, where the mosquitos’ lifespan is longer and where they prefer to bite humans rather than other animals. The long lifespan and strong human-biting habit of the African vector species is the main reason why approximately 90% of the world's malaria cases are in Africa.
For a long time, the only way to prevent Malaria was to focus on the mosquitos. Control of the insect vectors helps keep the disease in check. Destroying mosquito populations or reducing insect bites by measures such as using effective mosquito nets at night have also proven effective in reducing malaria transmission.
Last edited: 29 July 2022 10:23