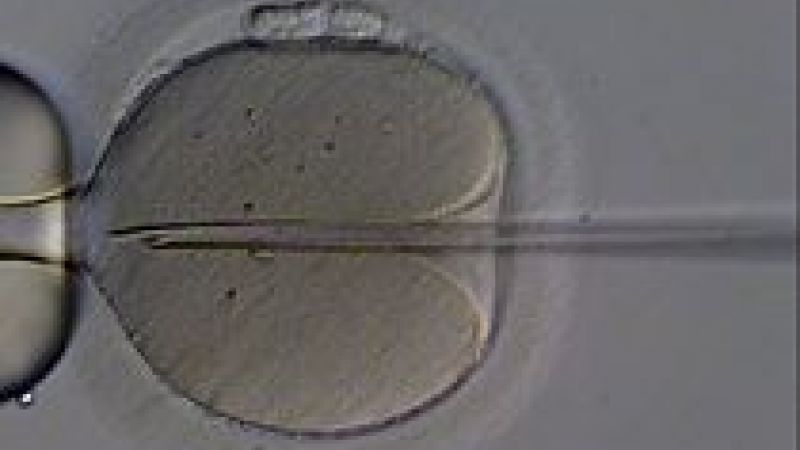
In a historic vote, British MPs in the House of Commons voted in favour of allowing the creation of babies with DNA from two women and one man – the so-called “three-person IVF”. By a vote of 382 for, to 128 against, the UK looks set to become the first country in the world to allow this new technique, which aims to prevent mitochondrial diseases being passed from mother to child. All that is necessary now is for the House of Lords to approve the treatment. According to the BBC, the first baby could be born using this technique next year.
While the idea of 3-person IVF or 3-parent babies brings up the images of two mums and a dad, the reality is that the baby would have 50% of the father’s DNA, 49.9% of the mother’s DNA, and 0.1% of a donor woman’s DNA. This 0.1% aims to fix defective mitochondria – small structures in the cell that provide the cell with chemical energy. Mitochondria contain 37 coding genes, compared with the 20,000 coding genes contained within the nucleus of the cell. The mitochondrial genes are always inherited from the mother’s side, so any genome mutation will always be passed onto her children. Defective mitochondria can lead to genetic diseases resulting in brain damage, muscle wasting or heart failure.
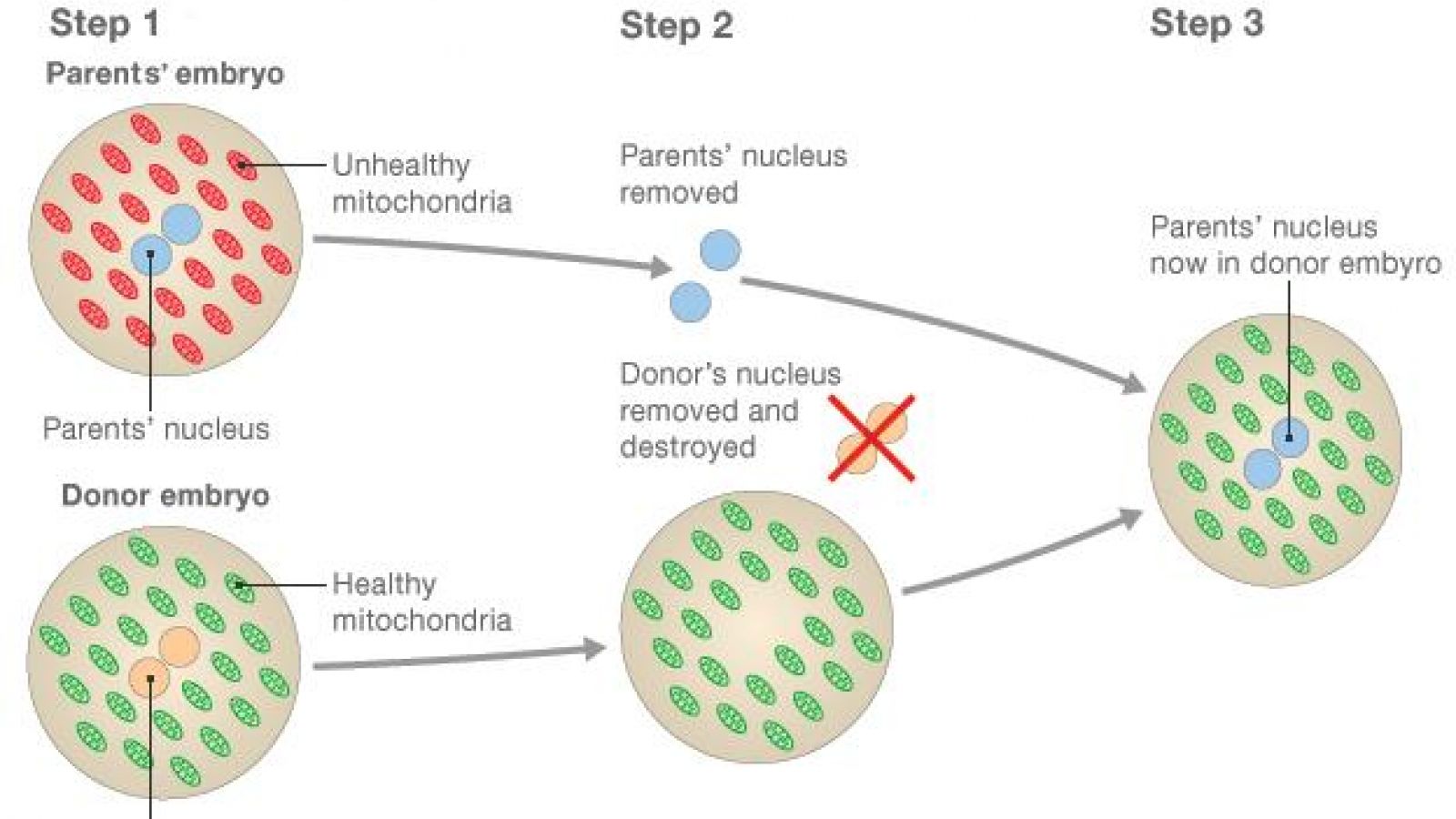
There are two methods of 3-person IVF, one which involves getting a donor embryo (fertilised egg) and replacing the donor’s nucleus with the nucleus from the parents’ embryo – this allows the parents’ embryo to grow in an environment with healthy mitochondria.

The second method is similar, but involves removing the genetic material from both a mother's and donor’s eggs, then placing the mother’s genetic material in the donor egg (which has healthy mitochondria). This can then be fertilised by the father’s sperm.

A 2012 paper in Nature (1) published by Dr Mitalipov’s team at Oregon Health and Sciences University (OHSU), USA, demonstrated that it was possible to replace faulty mitochondria of a human egg cell before fertilisation and create a healthy-looking human embryo.
Mitalipov is clear that this would not have been possible had the technique not been pioneered in primates. His team worked at OHSU’s attached primate facility – Oregon National Primate Research Centre – to demonstrate spindle-chromosomal complex transfer in primates (2). The team created 3 healthy baby macaque infants using the method, with many refinements necessary to the technique also developed using primates.
The more recent paper, which noted the potential for success in humans, also took time to note that the baby monkeys born through spindle-chromosomal complex transfer were in good health and developing normally after 3 years.
Other key research in rhesus macaques involved finding out whether the method would work using frozen egg cells – essential for clinical viability. Dr Paul Browne writes:
“More surprisingly they also found to their surprise that while the spindle–chromosomal complex could withstand prior cryopreservation the technique failed when the egg into which the spindle-chromosal complex is transferred had been frozen – indicating that most of the damage to the frozen egg is to its cytoplasm, rather than to the nucleus as had previously been thought, a discovery that may have wider implications for the future improvement of human egg cryopreservation and IVF techniques.”
It is great to see Britain leading the way in the take-up of new scientific techniques that will benefit human health, and we should appreciate the animals which made this technique possible.
1. Masahito Tachibana, Paula Amato, Michelle Sparman, JoyWoodward, Dario Melguizo Sanchis, Hong Ma, Nuria Marti Gutierrez, Rebecca Tippner-Hedges, Eunju Kang, Hyo-Sang Lee, Cathy Ramsey, Keith Masterson, David Battaglia, David Lee, Diana Wu, Jeffrey Jensen, Phillip Patton, Sumita Gokhale, Richard Stouffer& Shoukhrat Mitalipov “Towards germline gene therapy of inherited mitochondrial diseases” Nature. Published online 24 Oct 2012, doi:10.1038/nature11647
2. Masahito Tachibana, Michelle Sparman, Hathaitip Sritanaudomchai, Hong Ma, Lisa Clepper, Joy Woodward, Ying Li, Cathy Ramsey, Olena Kolotushkina & Shoukhrat Mitalipov “Mitochondrial gene replacement in primate offspring and embryonic stem cells” Nature 461, 367-372 (2009) doi:10.1038/nature08368
Last edited: 22 July 2025 12:09