Penicillin
Florey and Chain first tested the effects of penicillin in mice in 1940. By 1941, penicillin was being used to treat dying soldiers. This research won the Nobel Prize in 1945.
Sir Howard Florey helped to develop penicillin, which had been discovered by Sir Alexander Fleming in 1928 in the form of a rare mould called Pencillium rubens growing in his London lab. It fell to Florey and his Oxford team, which included Norman Heatley and Ernest Chain, to produce the drug as a stable powder and to show that it could cure mice infected by bacteria. The team then needed the assistance of the US Department of Agriculture to help refine production techniques and find a more effective strain of mould, and US-based drug companies to further refine and mass-produce penicillin in time for the D-Day landings of World War 2.
“I did not invent penicillin. Nature did that. I only discovered it by accident.” - Alexander Fleming
Read our article "Did animal tests delay pencillin by ten years?"
Accidental discovery and deliberate testing
In 1928, Fleming began a series of experiments involving the common staphylococcal bacteria. Although legend has it that mould floated fortuitously in through an open window and landed on a petri dish full of the bacteria, the contamination may have come from another lab in the building that had collected mould spores from the houses of asthmatic patients, or may have simply been present in Fleming’s lab. No bacteriologist leaves their lab window open and unattended for weeks, but La Touche’s samples in the asthma lab downstairs were simply laid out on the bench top and both Fleming and La Touche never shut their doors. Equally, genetic sequencing in 2011 revealed that Fleming, La Touche, and subsequent classifications of the original spore had misclassified Penicillium rubens as a number of other sub-species of mould. What definitely happened was Fleming was going on holiday and stacked his (covered) spore plates on one side of his work bench so his new research assistant Stuart Craddock could work at his bench while he was away.

Fleming’s work bench
The petri dish had become contaminated with mould spores, maybe from the asthma lab, prior to being sealed in with the bacterial culture but also sat in ideal growing conditions for mould due to a fluke of unseasonably mild weather. Between July and August 1928 in London, the temperature only exceeded 20°C twice.
Upon returning from holiday, Fleming lifted the lid of the top dish and saw the following, which he photographed:
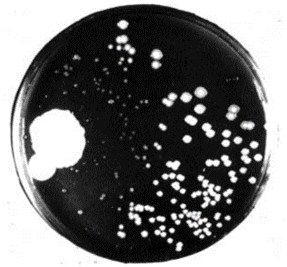
A photo of the dish, taken by Fleming.
He observed that the bacteria in proximity to the mould colonies were dying, as evidenced by the dissolving and clearing of the surrounding agar gel. He was able to isolate the mould and identified it as a member of the Penicillium genus.
He found it to be effective against all Gram-positive pathogens, which are responsible for diseases such as scarlet fever, pneumonia, gonorrhoea, meningitis and diphtheria. ‘Gram positive’ are bacteria classified by the colour they turn in a staining method developed by Hans Christian Gram. Fleming discerned that it was not the mould itself but some ‘juice’ it had produced that had killed the bacteria. He named the ‘mould juice’ penicillin.
Later, he would say:
“When I woke up just after dawn on September 28, 1928, I certainly didn’t plan to revolutionise all medicine by discovering the world’s first antibiotic, or bacteria killer. But I suppose that was exactly what I did.”
In 1929, he published his findings in the British Journal of Experimental Pathology.
Difficulties purifying penicillin
However, he encountered difficulties extracting and purifying his ‘mould juice’. He initially set his assistants, Craddock and ophthalmologist Frederick Ridley who’d joined Fleming in 1926, the difficult task of isolating pure penicillin from the juice. It proved to be very unstable, they were only able to prepare solutions of crude material to work with and they gave up in 1930. Fleming tried, unsuccessfully, to treat Craddock’s sinusitis with the penicillin broth in January 1929. Ridley used it to treat minor eye infections, and St Mary’s Hospital in Paddington, London, used it to treat infections and boils.
As Craddock and Ridley laboured on, Fleming escalated his attempts at enlisting help with purification, inviting the UK’s top chemists to try their hand at the task.
Several tried and failed, including famed biochemist Prof Harold Raistrick, then at the London School of Hygiene and Tropical Medicine, who had relevant experience in fermentation and microbiology. Raistrick was skilled at applying scientific knowledge to real-world applications from explosives to mining to medicines. However, he subsequently declared the “production of penicillin for therapeutic purposes…almost impossible.”
In fact, Lewis Holt, another chemist who also tried to purify it, came extremely close but used the wrong chemical ph and was unaware of the previous work done by Ridley that could have informed his approach. Equally, Ridley and Craddock did succeed in producing one batch of high potency penicillin, but inexplicably failed to undertake the mouse protection test that would have proven the worth of penicillin, as the test later would, years earlier.
First described in 1911, the mouse protection test was routinely used from 1927 and ultimately led to the introduction of sulphonamide (antibiotic) drugs in the 1930s. Fleming himself had injected a mouse and a rabbit with the penicillin, but it seems that was to check that it wasn’t toxic – the reason he didn’t infect the animals first is a mystery.
Interest in Fleming’s discovery had been muted from the beginning, and when Fleming talked of its medical importance at the Second International Congress of Microbiology held in 1936 no one believed him.
But this confluence of factors – the challenge of purification, scientists working on the same problem being unaware of the work of other scientists, the inexplicable decision not to use the mouse protection test and even Fleming’s lack of persuasiveness, meant that the potential of penicillin remained largely unrealised in the 1930s, even though Fleming’s notes show that he kept working on the problem right up until others took up the mantle.
In 1936, Fleming was lecturing medical students including Dr Bill Frankland, an allergist who was also responsible for the pollen count being part of the weather forecast. Dr Frankland died in April 2020 at the age of 108, but gave an interview to Imperial College London in 2018 in which he stated:
“It was [at a lecture] in 1936 that Fleming said ‘we have this new substance that I have designated penicillin, because it comes from a penicillin mould.. And when we are able to use it commercially, on humans, it will change the whole of medicine. He said there would be a revolution, but doctors will overuse it, and because bacteria have to survive - they are very, very clever - they will become resistant to it. I didn’t know what he was talking about at the time, it seemed all rather silly to me. But he had already realised this was going to happen”
Penicillin (finally) purified thanks to Heatley
In 1937, a team led by Howard Florey was searching for potential antibacterial agents. Penicillin was identified as a compound with potential, Fleming’s earlier findings were confirmed and team member Edward Abraham was the first to propose the correct structure of penicillin, having studied the molecule with Ernst Chain. Shortly after the team published its first results in 1940, Fleming telephoned Florey to say that he would be visiting within the next few days. When Chain heard that Fleming was coming, he remarked "Good God! I thought he was dead."
After this, it fell to team member Norman Heatley to be the latest biochemist to attempt purification.

Norman Heatley
Heatley was said to have possessed a natural gift for ingenuity and invention and devised a novel approach of back-extraction, transferring the active ingredient of penicillin back into water by changing its acidity, thus purifying the penicillin. It worked.
First animal trials of the purified penicillin
Heatley recorded the first animal trials, carried out on eight mice in May 1940, in his diary:
"After supper with some friends, I returned to the lab and met the professor to give a final dose of penicillin to two of the mice. The 'controls' were looking very sick, but the two treated mice seemed very well. I stayed at the lab until 3:45 a.m., by which time all four control animals were dead."
On returning home, he realised that in haste and darkness, he had put his underpants on back to front, and noted this in his diary too, adding "It really looks as if penicillin may be of practical importance."
Production problems, Heatley once again aces it
The paper they published detailing the experiment gained immediate interest, but the team were now severely limited by production. Gallons of mould broth was required to produce just a fingernail of penicillin. The team resorted to using bedpans, milk churns, food tins and even bathtubs to store the broth.
Again, it was Heatley who realised that the most effective vessel for this purpose was something like the porcelain bedpans in use at the Radcliffe Infirmary. These were in short supply due to the ongoing World War II, so Heatley designed a modified version.

Image: science museum
With penicillin now in successful albeit slow production, the Oxford laboratory had now become a penicillin factory. Six women, known as the ‘Penicillin Girls’ were employed to tend to the fermenting broth and ‘farm’ a few precious milligrams of penicillin from it every week.

Two of the six ‘Penicillin girls’ that included: Ruth Callow, Claire Inayat, Betty Cooke, Peggy Gardner, Megan Lancaster and Patricia McKegney.
First human trial and the Pee Patrol
In 1941, the consequences of the teams’ production problems and shortage of penicillin became apparent with the first human trial of penicillin.
Albert Alexander, a 43-year-old policeman, had developed a life-threatening infection from a cut. He was dosed with 160mg of penicillin and within 24 hours his infection was under control and his appetite returned. However, the supply of penicillin ran out after 5 days and Albert’s infection returned. Around 80% of a dose of penicillin is excreted in urine so the team, desperate for more of the drug, took to collecting and filtering his urine. For the final doses. Dr Ethel Florey, a supervisor for the clinical trials, was regularly observed on the ’P-Patrol’, cycling to patients to collect their urine.
Sadly, it wasn’t enough and the patient died a month later.
Corn in the USA
It was clear that production needed to scale up from what it could deliver at the time, but UK pharmaceutical companies were too caught up in the war effort to help, so, in June 1941, Florey took penicillin to the US.
In Peoria, Illinois, a new team was set up in the Department of Agriculture’s research laboratory. They utilised their expertise in fermentation and designed new techniques using deep fermentation tanks to make the purification of penicillin as efficient as possible.
The lab in Peoria had an abundance of corn-steep liquor, a by-product of corn starch. They discovered that when added to the mould broth, the yield of penicillin increased exponentially. The high concentration of sugars, amino acids and nitrogen provided an excellent environment for mould fermentation.
Moldy Mary and the rotten cantaloupe
They started a global search for strains of mould with higher percentages of penicillin and soil samples were sent in from around the world, many from foreign US military bases. However, the solution was found closer to home. Mary Hunt, an assistant at the Peoria lab, was in charge of collating the samples and was known affectionately as ‘Moldy Mary’. She either bought, or was given, a rotting cantaloupe melon from a local farmer’s market. The mould produced six times more penicillin than Fleming’s original strain.
around the world, many from foreign US military bases. However, the solution was found closer to home. Mary Hunt, an assistant at the Peoria lab, was in charge of collating the samples and was known affectionately as ‘Moldy Mary’. She either bought, or was given, a rotting cantaloupe melon from a local farmer’s market. The mould produced six times more penicillin than Fleming’s original strain.
Further innovations boosted the amount and quality of penicillin. G. Raymond Rettew made a significant contribution through his techniques to produce commercial quantities of penicillin, wherein he combined his knowledge of mushroom spawn with the function of the Sharples Cream Separator. His lab soon produced most of the world’s penicillin.
US Pharmaceutical companies were initially reluctant to commit to large scale penicillin production. However, by the end of 1941 the US joined the Second World War. In the UK, the War Cabinet created the Penicillin Committee in April 1943 and in July of that year the US War Production Board drew up plans for the mass distribution of penicillin to Allied troops, and that involved more than 20 pharmaceutical companies, as their advertising from the time will repeatedly tell you.

By 1943, the US had sufficient penicillin stocks to satisfy the demands of the Armed Forces of the United States, as well as their Allies.
Lessons and myths
Activists and politicians opposed to using animals in research sometimes claim that penicillin was ‘delayed by 10 years because animal tests were misleading’. This is clearly very wrong and more or less an inversion of the truth since, had the mouse protection test been undertaken by Craddock and Findley, then the development work critical to making it a lifesaving drug would likely have started years sooner and saved even more lives.
More to the point, there were no misleading animal results and the attempted treatment of humans started almost immediately.
The less harmful myth of the first penicillin mould floating through an open window is simply implausible, involving a window being left open for several months in cool weather, while ignoring literal trays of mould spores left out in a nearby room.
The lessons are manifold. Firstly, it took a thousand people to make this story happen. We’ve alighted upon some of the key moments, but none of it could have come into being without the efforts of everyone, and every sector, involved.
Pharmaceutical companies, animal and nonanimal methods, curious scientists, jobbing nurses, unsung geniuses – all of it played into how things played out. In hindsight, it’s easy to point out ways the success could have been expedited, but in the fog of the present it’s easy to see how that doesn’t happen.
Perhaps another major lesson was the importance of being aware of the work of others, whether that was Lewis Holt’s ignorance of Ridley’s work, or Florey’s team looking back for discoveries that would take extra work to get from lab bench to bedside.



