Animal Research in the papers
The Guardian: Experts urge health regulators to approve ‘turning point’ dementia drugs
Organisations, including Concordat Signatory Alzheimer’s Research UK, are urging health regulators to approve two new Alzheimer’s drugs, donanemab and lecanemab. The final results from Eli Lilly’s large scale human clinical trial confirmed that the drug donanemab slowed cognitive decline by 35%. Last year, clinical trial results were published that showed another drug, lecanemab, reduced the rate of decline by 27%. Following last year’s trial results, we wrote about the animal testing that was required to develop lecanemab.
Both drugs use monoclonal antibodies to clear the amyloid plaques that have been linked to Alzheimer’s disease. In development for more than a decade, pre-clinical tests in mice laid the groundwork for scientists to pinpoint the right antibodies to use and to prove that the drug is capable of clearing amyloid plaques in the brain before moving to clinical trials.
Read Experts urge health regulators to approve ‘turning point’ dementia drugs
Extra sources:
AlzForum: Donanemab
News from UAR Members and Concordat Signatories
University of Cambridge
Unborn babies use ‘greedy’ gene from dads to ‘remote-control’ mums into feeding them extra food
A study in mice has found that foetuses use a copy of a gene inherited from their dad to force their mum to release as much nutrition as possible during pregnancy. The recently published study examines how the placenta communicates with the mother through the release of hormones so she will accommodate her baby’s growth.
Read: Unborn babies use ‘greedy’ gene from dads to ‘remote-control’ mums into feeding them extra food
Compass Pathways
The first results of a human clinical study on treating anorexia with COMP360 psilocybin have found that 40% of participants experienced significant reductions in the abnormal psychological symptoms of anorexia as well as a reduction in concerns around weight and body shape.
In 2021, we wrote about the ongoing research on whether COMP360 psilocybin can treat depression and the animal tests in mice and rats.
GSK
New research published in Science highlights potential of a naturally occurring bacterium to help eradicate malaria
Scientists at GSK have discovered that a strain of Delftia tsuruhatensis bacterium inhibits P. falciparum, a malaria parasite found in mosquitoes. The discovery has led to a collaboration with the Johns Hopkins Malaria Research Institute that confirms that the bacteria drastically reduces the amount of malaria parasites in the mosquitoes, potentially reducing transmission to humans significantly.
The University of Manchester
Research discovers key cause of restricted blood flow to the brain in vascular dementia
New research in mice has shown that high blood pressure causes changes to arteries in the brain, a process that leads to vascular dementia. The research, led by University of Manchester scientists, funded by the British Heart Foundation, has uncovered a route to developing the first ever drug treatments for vascular dementia that directly target a cause of the condition.
Read: Research discovers key cause of restricted blood flow to the brain in vascular dementia
Swansea University
New study: type 2 diabetes drug could treat autoimmune disorders
Researchers at Swansea University have discovered that a drug commonly used to treat type 2 diabetes may be suitable for treating autoimmune disorders. The drug, canagliflozin (also known as Invokana), could be used to treat autoimmune disorders such as rheumatoid arthritis and systemic lupus erythematosus as it targets T-cells, which form an essential component of the immune system.
Historically, canagliflozin was tested on mice, rats, dogs, and humans during its development as a drug to treat diabetes.
Read: New study: type 2 diabetes drug could treat autoimmune disorders
Extra sources:
Drug Metabolism and Disposition Journal: Metabolism and Excretion of Canagliflozin in Mice, Rats, Dogs, and Humans
UCL
UCL backed pancreatic cancer therapy cleared for clinical trials
A new pancreatic drug, QN-302, that has been in development at UCL has been given the go-ahead to begin stage one clinical trials in humans by the USA’s FDA. The first patients to be included in the trial with QN-302 are expected to begin receiving the therapy as early as autumn 2023.
The therapy targets the unusual signal sequence (abnormalities) that are present in high levels in many cancer-associated genes. By blocking these signal sequences, the drug has been shown to stop cancer growth in pre-clinical models. Pre-clinical tests using mice have so far been used to show that the therapy does not produce adverse toxicological effects.
Read: UCL backed pancreatic cancer therapy cleared for clinical trials
Extra sources:
Yahoo: Encouraging Preclinical Data Presented for QN-302 and RAS Inhibitor
The Babraham Institute
Being open to openness: my personal experience with public engagement
A new blog post from an experienced animal technician at the Babraham Institute discusses their experience with openness and transparency around animal research. In the blog, they cover their perspective as an animal technician, what it means to be part of the Institute’s culture of openness, and how they have approached public engagement at the Institute.
Read: Being open to openness: my personal experience with public engagement

This image from the Babraham Insitute shows a technician holding a mouse with black fur.
Newcastle University
The ovarian cancer drug developed by Newcastle scientists
An ovarian cancer drug developed at Newcastle University which was approved for use in the NHS in 2019, is now directly benefitting patients. The drug Rubraca, offers patients a treatment option to help prevent cancer growth, delaying the need for further chemotherapy and the associated side-effects.
Rubraca was tested on xenograft mouse models of breast cancer to determine efficacy and dosage.
Read: The ovarian cancer drug developed by Newcastle scientists
Extra sources:
European Medicines Agency: Assessment report Rubraca
Charles River Laboratories – Eureka Blog
The Evolving Mouse Model in Cancer Research
A new blog post from Charles River Laboratories takes a deep dive into the use of mouse models in cancer research. In the blog they cover, how oncology mouse models are advancing, the limitations of current animal models, and the future of mouse models and cancer research.
Read: The Evolving Mouse Model in Cancer Research
The Pirbright Institute
World Zoonoses Day: How Pirbright is protecting you
A new video from The Pirbright Institute looks at zoonosis, the diseases that can be passed between humans and animals, and how their research aims to protect society from the risk of zoonotic disease.
3Rs – Replacement, Refinement, and Reduction of Animals in Research
The University of Edinburgh
New tech is step towards lab-grown blood vessels
A new technology that creates ultra-thin layers of human cells in tube-like structures could lead to the development of lifelike blood vessels and intestines in the lab. Naturally, this would help to reduce the need for animal testing and testing using animal tissues or cells.
Read New tech is step towards lab-grown blood vessels
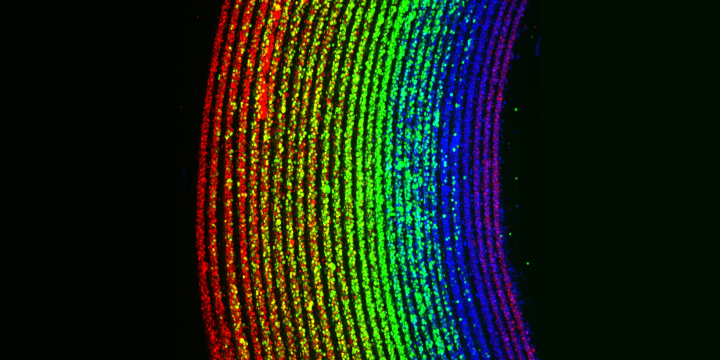
The University of Edinburgh: A microscopic rainbow created using ultra-thin layers of human cells and the new RIFLE biofabrication technology
The University of Nottingham
Researchers develop new platform that could replace the use of animals in research
A new 3D cell culture platform, provided by the university’s spin-out company PeptiMatrix, aims to replace the use of animals in research.
With the support of the NC3Rs, BBSRC, and EPSRC, researchers at the University of Nottingham have developed a fully synthetic self-assembling peptide hydrogel (SAPH) platform, which aims to address all the current shortcomings of available in vitro models.
Read: Researchers develop new platform that could replace the use of animals in research
News from the animal research sector
UK Government
Advice on the use of the forced swim test
The UK government commissioned the Animals in Science Committee (ASC) to provide advice on policy questions related to the forced swim test. The ASC’s guidance has been published and states that:
- The Forced Swim Test should only be used for screening potential antidepressants and studying the effects of stress on the nervous system.
- The Forced Swim Test should not be used as a model of depression or to study depression-like behaviour.
- When considering the availability of alternative methods, the ASC did not find any suitable methods to replace justifiable use of the Forced Swim Test. However, replacement technologies are in development and may be available for use in the future.
Read: Advice on the use of the forced swim test
UKRI
Position statement on research and innovation involving animals
“New position statement harmonises expectations for the involvement of animals in research and innovation across the whole of UK Research and Innovation (UKRI).”
Read: Position statement on research and innovation involving animals
Australian Transparency Agreement
Openness Agreement on Animal Research and Teaching in Australia
Australian working group ANZCCART, supported by UAR Oceania, have officially launched their new Openness Agreement on Animal Research and Teaching Australia. The first 30 organisations to sign the agreement include universities, medical research institutes and other institutions associated with the use of animals in biomedical research and teaching. Australia’s new openness agreement is the 10th of its kind across the world and was based on the UK’s Concordat on Openness on Animal Research which was launched in May 2014.
Bella Lear, Chief Executive of UAR Oceania, said: "This commitment to openness in animal research supports the scrutiny and forward-thinking that will ultimately drive better science and build better futures in Australia, and around the world."
Read: Openness Agreement on Animal Research and Teaching in Australia
Extra sources:
EARA: Australia pledges greater transparency in animal research
Canadian Government
Health Canada Announces the End of Cosmetic Animal Testing in Canada
The Government of Canada is taking action to ban cosmetic animal testing while ensuring the continued protection of human health and safety of all Canadians. This is welcome news as many countries are now looking to overhaul their regulations around the use of animals in cosmetic testing, following the ban in the UK (1996) and across the EU (2013).
Read: Health Canada Announces the End of Cosmetic Animal Testing in Canada
For information on the cosmetic testing ban in the UK: Cosmetic testing on animals
Read more animal research news here
Last edited: 27 September 2023 13:45




